Advanced Mfg in Pharma: Where Does It Play?
How does advanced manufacturing in pharma, such as continuous manufacturing, AI-enabled manufacturing, or distributed manufacturing factor into the current investment activity and policy moves to increase US-based pharma manufacturing? DCAT Value Chain Insights takes an inside look at the latest developments.
By Patricia Van Arnum, Editorial Director, DCAT, pvanarnum@dcat.org
Regulatory framework and advanced manufacturing
Adopting innovative approaches to manufacturing may present both technical and regulatory challenges. On the regulatory front, pharmaceutical companies may have concerns that developing such technologies could result in delays while novel regulatory challenges are considered. This is especially true while FDA assessors familiarize themselves with new technologies and determine how they may be evaluated within the existing regulatory framework. To address these concerns, the Office of Pharmaceutical Quality under FDA’s Center for Drug Evaluation and Research (CDER) created the Emerging Technology Program in 2014 as a collaborative program where industry representatives can meet with Emerging Technology Team members to discuss, identify, and resolve potential technical and regulatory issues regarding the development and implementation of a novel technology prior to filing a regulatory submission.
Following a 2021 report by the National Academies of Sciences, Engineering, and Medicine that identified innovations CDER is likely to encounter that have the potential to advance pharmaceutical manufacturing and with further engagement with stakeholders through the Emerging Technology Program, CDER established the Framework for Regulatory Advanced Manufacturing Evaluation (FRAME) initiative to prepare a regulatory framework to support the adoption of advanced manufacturing technologies. The FRAME initiative prioritized four technologies (see Figure 1) and as outlined below.
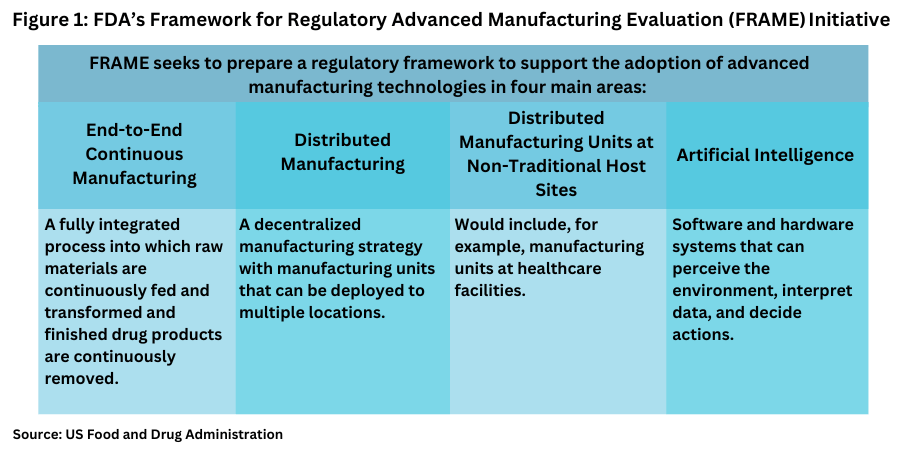
End-to-End Continuous Manufacturing. A fully integrated process into which raw materials are continuously fed and transformed and finished drug products are continuously removed.
Distributed Manufacturing. A decentralized manufacturing strategy that has manufacturing units that can be deployed to multiple locations.
Distributed Manufacturing Units at Non-Traditional Host Sites: This would include, for example, distributed manufacturing units at healthcare facilities.
Artificial Intelligence (AI): Using software and hardware systems that can perceive the environment, interpret data, and decide actions.
Advanced manufacturing projects
To date, the current Administration has focused on providing incentives to reshore/onshore domestic pharmaceutical manufacturing in the US through its tariff/trade policies and through administrative action to facilitate reviews of US-based pharma manufacturing facilities. It has also provided some funding for specific projects in advanced manufacturing. In May (May 2025), the US government launched four projects aimed at advancing pharmaceutical manufacturing in the US using AI, machine learning, and informatics for certain generic drugs. The program, called Equip-A-Pharma, is a collaboration between the US Department of Health and Human Services (HHS), the Administration for Strategic Preparedness and Response (ASPR), the Defense Advanced Research Projects Agency (DARPA), and the private sector.
Over the next year (as reported on May 15, 2025), each company and its partners will aim to show how their technologies can potentially make active pharmaceutical ingredients and specific finished drug formulations at the point-of-care. All of the partners in these projects are expected to submit abbreviated new drug applications to the US Food and Drug Administration within a year (as reported on May 15, 2025) as a result of leveraging the government-supported development work.
“Manufacturing medicines from start to finish in the United States ensures a secure supply of essential medications,” said HHS Principal Deputy Assistant Secretary for Preparedness and Response John Knox, in a May 15, 2025, statement in commenting on the launch of the program. “Traditional pharmaceutical manufacturing is often too rigid and slow to adapt to changing demands, especially during national emergencies. We’re launching projects aimed at completely changing the approach not just to bring pharmaceutical manufacturing back to the US but to do it better.”
By producing active pharmaceutical ingredients and finished medications at the point of care, such as a hospital, these types of technologies can strengthen domestic supply chains, ASPR says, and that this approach could mitigate drug shortages, enhance national health security and improve resilience. These projects address US-based manufacturing of eight sterile injectable or oral medications and the active pharmaceutical ingredients for these medicines. The partners conducting the projects are Battelle Memorial Institute and Aprecia; BrightPath Laboratories; Rutgers University; and the Mark Cuban Cost Plus Drug Company.
Partners Battelle Memorial Institute and Aprecia are integrating advanced process analytics and machine learning that allows for real-time quality assessment and supports a digital approach to generating regulatory submissions. The companies are using their own technologies to produce 3-D printed oral solid dosage forms (tablets) of levetiracetam to treat epilepsy or other seizures, and linezolid to treat gram-positive infections such as bacterial pneumonia.
Bright Path is demonstrating technology for continuous flow manufacturing to produce lidocaine HCl, a local anesthetic, and carboplatin, a chemotherapy drug. The project is expected to show enhanced efficiency, scalability, adaptability of the technology, rapid reconfiguration between products with minimal downtime, and a real-time digital approach to generating regulatory submissions. The technology integrates advanced process informatics and in-process metrology.
Rutgers University will demonstrate manufacturing of registration batches for two drugs, bupivacaine HCl, an anesthetic, and albuterol sulfate, used to treat asthma, bronchitis, emphysema or other lung conditions. Rutgers has been working with ASPR’s Center for Industrial Base Management and Supply Chain and DARPA to develop a manufacturing platform and generate data sets using new AI tools that can inform future regulatory submissions and rapid release of products.
The Mark Cuban Cost Plus Drug Company is employing a highly automated and flexible manufacturing platform that integrates AI and machine learning for real-time quality assessment and process optimization. The company is demonstrating use of the platform to process active pharmaceutical ingredients and the finished medication doses rapidly with in-line metrology to produce lidocaine, a widely used anesthetic, and diltiazem, a calcium channel blocker used to treat heart conditions. The system uses AI-driven informatics, is scalable and adaptable, and can be used by operators with minimal training to maintain product quality.
Other industry projects
In January (January 2025), Purdue University, in collaboration with Eli Lilly and Company and Merck & Co., announced the launch of the Young Institute Pharmaceutical Manufacturing Consortium, a collaboration with a focus on sterile injectables and innovative aseptic manufacturing technology. Elizabeth Topp, Director of the Young Institute, will serve as the new consortium’s Director. She is a professor in the Department of Industrial and Molecular Pharmaceutics and the Davidson School of Chemical Engineering at Purdue University with expertise in improving the shelf life and stability of pharmaceuticals.
The members of the new consortium say they anticipate the consortium will attract broad participation throughout the sector from other pharmaceutical companies, pharma contract manufacturers, equipment manufacturers, start-ups, and venture capital firms. The consortium will look to create systems equipped with automated visual inspection and in-line process and product-quality monitoring, all of which will require a new generation of skilled pharmaceutical manufacturing engineers and scientists. The consortium will seek to advance manufacturing by developing innovative technologies, autonomous systems, smart AI, and related digital technologies, together with industrially relevant education and training. The collaboration also underscores a commitment to onshoring pharmaceutical manufacturing while bolstering domestic production.
In addition, in May (May 2025), Purdue University and a coalition of leaders in AI, pharmaceutical manufacturing, and public policy launched a national effort to onshore pharmaceutical manufacturing in the US by applying AI and advanced manufacturing technologies. Led by Purdue, the Information Technology and Innovation Foundation, and the National Institute for Pharmaceutical Technology and Education, coalition representatives signed a collaborative accord that outlined concrete objectives to lower production costs, improve quality control, build a geographically distributed manufacturing base, accelerate regulatory pathways, and train a new generation of skilled talent to lead in AI-enabled pharmaceutical innovation as a means to strengthen US-based pharmaceutical manufacturing. Other signatories to the accord included Eli Lilly and Company, Evonik, Phlow Corporation, Google, and Blackstone Life Sciences, among others.








