FDA Begins Pilot for Speeding Drug Reviews with A Priority for Reshoring Mfg
FDA is starting a new pilot program to reduce review times from 10-12 months to 1-2 months for certain drugs, with one priority given to drugs that reshore/onshore manufacturing to the US.
By Patricia Van Arnum, Editorial Director, DCAT, pvanarnum@dcat.org
New priority review program
The US Food and Drug Administration (FDA) is moving forward with a pilot program in which the agency is seeing to reduce the agency’s drug review times for standard review to 1 to 2 months for certain novel drugs. Called the Commissioner’s National Priority Voucher (CNPV), the voucher would apply to drugs participating in a novel drug FDA priority program and that meet certain criteria, including consideration that the drug is being manufactured in the US.
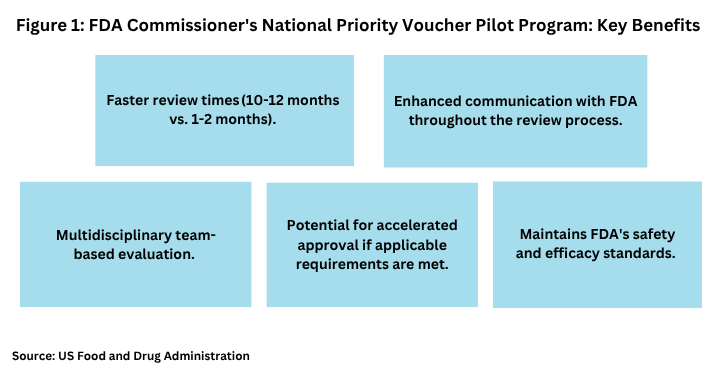
FDA points to several key benefits to participate in the program (see Figure 1). Chief among them is reducing the standard review time by FDA for qualifying drugs from 10 to 12 months to 1 to 2 months. It would achieve that by using experts from FDA offices for a team-based review rather than using the standard review system of a drug application being sent to numerous FDA offices. Announced last month (June 2025), the new program uses a collaborative tumor-board style review process to accelerate approvals for companies aligned with certain US health priorities, which among other priorities includes consideration that the drug is being manufactured in the US. Earlier this month (July 2025), FDA opened the process for a limited number of drug and biologic developers to participate in the CNPV pilot program.
Aligning with US health priorities
FDA says the CPNV program is designed to provide accelerated review times for certain drugs and biological products that are aligned with US national health priorities (see Figure 2).
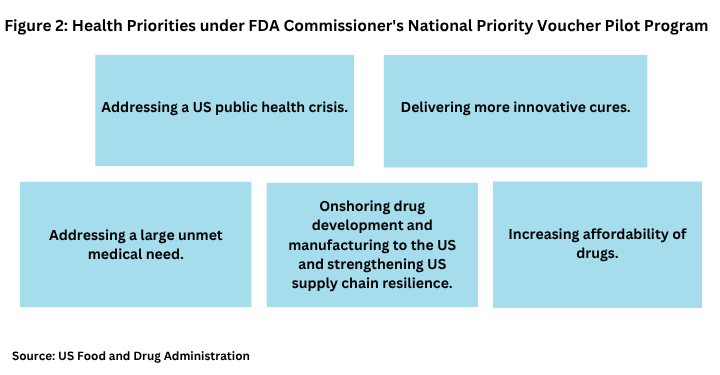
Specific priorities include:
Addressing a US public health crisis. FDA says an example could include developing a universal flu vaccine that could provide broad protection against multiple strains of influenza, including those with pandemic potential.
Delivering more innovative cures. FDA says the focus for this priority is transformative impact that exceeds the threshold for breakthrough therapy designation. Breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat a serious condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
Addressing a large unmet medical need. This includes a condition that available therapies do not adequately diagnose or treat, including drugs to treat or prevent rare diseases or addressing chronic disease.
Onshoring drug development and manufacturing to the US and strengthen US supply-chain resiliency. Under this priority, examples could include companies with new manufacturing establishments that shift manufacturing of essential medicines (such as generic sterile injectables) from foreign facilities to the US or a clinical trial that maintains robust US enrollment to support generalizability for Americans against the US standard of care.
Increasing affordability of drugs. FDA says this could include a company that lowers the US price of a drug or drugs consistent with most-favored -nation pricing or reduces other downstream medical utilization to lower overall healthcare costs. Most-favored-nation drug refers to a policy proposal that the price of a drug in the US would be set no higher than the price of that drug in comparable countries, mostly developed nations.
How the CNPV would work
To participate in the CNPV program, a company must demonstrate alignment with one or more of the program priorities (see Figure 2 above). Companies are limited to submitting one application. Vouchers can be granted by FDA for review of a company’s specific drug or be granted to a company as an undesignated voucher, allowing a company to use the voucher for review of a pre-market application for a drug at the company’s discretion subject to consistency with the program’s objectives.
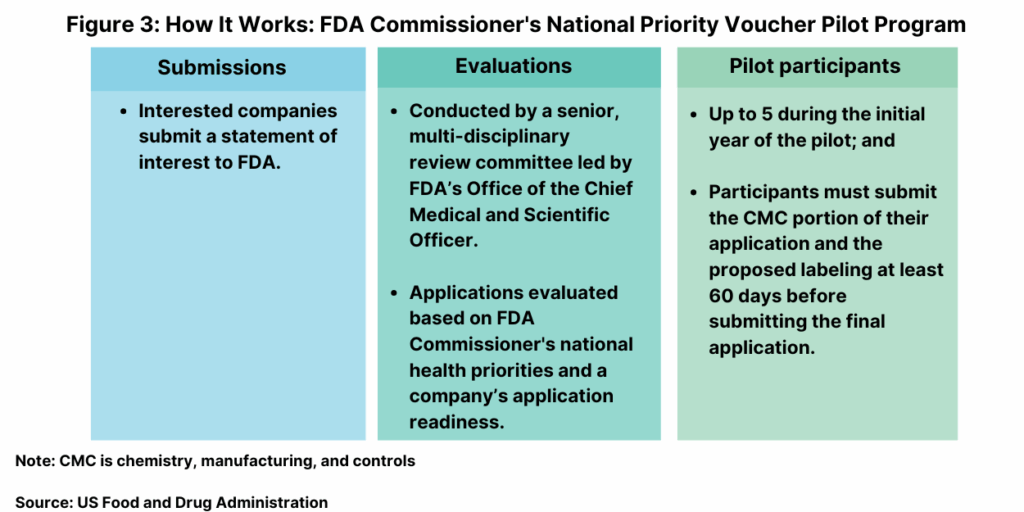
The program submissions will be evaluated by a senior, multi-disciplinary review committee led by FDA’s Office of the Chief Medical and Scientific Officer. The council intends to select pilot participants, with no more than five during the initial year, based on the FDA Commissioner’s national health priorities (see Figure 2 above) and application readiness (e.g., companies who demonstrate having the ability to move forward towards a marketing application). The council will select scientific and medical experts from relevant FDA offices and divisions for a team-based priority review.
To qualify to participate, sponsors must submit the chemistry, manufacturing, and controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application (see Figure 3 above). Sponsors must also be available for ongoing communication with prompt responses to FDA inquiries during the CNPV review. FDA says it reserves the right to extend the review window if the data or application components submitted are insufficient or incomplete, if the results of pivotal trial(s) are ambiguous, or if the review is particularly complex. Companies selected for the program will be issued a voucher entitling the company to benefits including enhanced communications and rolling review to allow for a shortened review time.







