Value-Added Medicines: A Call in the EU for a New Drug Class
Akin to the 505 (B)(2) pathway in the US, Medicines for Europe is calling on EU authorities to recognize value-added medicines as separate group of medicines in EU legislation with new approval procedures, innovation frameworks and reimbursement processes.
Seeking a regulatory pathway for value-added medicines
The Value Added Medicines Sector Group at Medicines for Europe, which represents generics and biosimilars companies in Europe, is seeking reforms in the European Union’s (EU) pharmaceutical legislation to recognize value-added medicines as a separate group of medicines and with that recognition, to develop specific frameworks for their regulation and reimbursement as a means to encourage innovation of off-patent molecules.
Such a regulatory framework would support the innovation of off-patent molecules akin to the 505(b)(2) regulatory pathway in the US. A 505(b)(2) application is a type of new drug application used to obtain the approval of a new drug that contains similar active ingredients to a previously approved drug and is used for changes in dosage form, strength, route of administration, formulation, dosing regimen, or new indication. The Value Added Medicines Sector Group at Medicines for Europe argues that no such similar pathway exists in the EU and is making several recommendations to create a regulatory pathway, set forth intellectual property protection requirements, and establish a reimbursement framework that would support innovation in off-patent molecules. Such measures would establish “value-added medicines” as a separate class of medicine and encourage innovation in formulation and drug delivery for existing drugs.
“Today, more than ever, there is a need for smart and sustainable healthcare solutions that can effectively address public health needs in a safe, timely and affordable way,” said Arun Narayan, Head of Global Commercial Development, Viatris, and Chair of the Value Added Medicines Sector Group at Medicines for Europe, in a November 30, 2022 statement. “Value Added Medicines can deliver in every aspect of these healthcare needs. But the lack of a supportive legislative and regulatory framework in Europe blocks Value Added Medicines from reaching patients. We believe that the revision of the EU pharmaceutical legislation provides a unique opportunity to address this gap, and we call for a suitable and robust framework for Value Added Medicines provided for in the new EU pharmaceutical legislation to help address both, the healthcare systems’ challenges and the patients’ needs.”
The Value Added Medicines Group first issued its call and recommendations for establishing value-added medicines as a separate class of medicines in the EU in November 2021 following the adoption of the Pharmaceutical Strategy for Europe, an EU-wide initiative that sets the strategic priorities for healthcare and pharmaceutical policy in the EU, by the European Commission in late November 2020, The Pharmaceutical Strategy for Europe sets both legislative and non-legislative action to achieve four main goals: (1) ensuring access to affordable medicines and addressing unmet medical needs (in the areas of antimicrobial resistance and rare diseases, for example); (2) supporting competitiveness, innovation, and sustainability of the EU’s pharmaceutical industry and the development of high quality, safe, effective, and greener medicines; (3) enhancing crisis preparedness and response mechanisms, building diversified and secure supply chains, and addressing medicines shortages; and (4) ensuring a strong EU voice in the world by promoting a high level of quality, efficacy and safety standards.
At the Value Added Medicines Conference held in Brussels late last month (November 2022), the Value Added Medicines Group of Medicines for Europe reiterated its call for reforms in EU pharmaceutical legislation to establish value-added medicines as a separate class of medicines as a means to stimulate innovation across the lifecycle of medicines and especially on off-patent molecules. To support the EU’s Pharmaceutical Strategy for Europe, the group says that value-added medicines present an opportunity to innovate on off-patent molecules and to bring treatments for indications that have no approved therapies and reduce unmet medical need (repurposing/repositioning), build on existing medicine to allow for patient-centric design or address healthcare inefficiencies (drug reformulation) and combine medicine and different services that can substantially improve treatment outcomes (complex combinations).
Figure 1 outlines the three main recommendations by the Value Added Medicines Sector Group of Medicines for Europe to support the development of value-added medicines in the EU. These are: (1) design a fit-for-purpose regulatory framework that will enable clarity early in development; (2) recognize value-added medicines as a category of innovation with proportionate incentives; and (3) recognize and define value for healthcare systems. These measures are further outlined below.
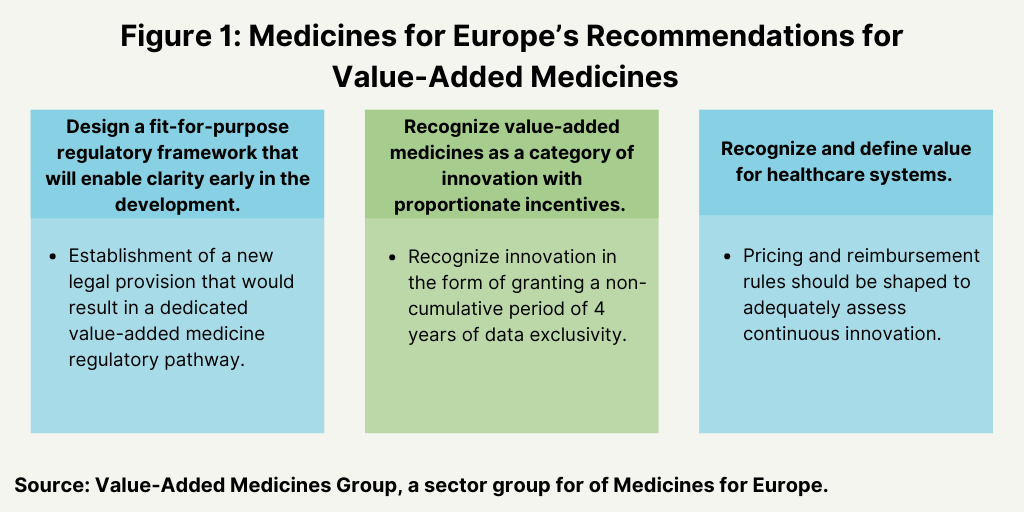
Fit-for-purpose regulatory framework. Medicines for Europe’s Value Added Medicines Group is recommending the establishment of a new legal provision that would result in a dedicated value-added medicine regulatory pathway. Such a dedicated regulatory pathway would include new, pragmatic methods of evidence generation and assessment for affordable and sustainable continuous innovation. This regulatory process would be accompanied by early dialogue with regulators, increased cooperation between all healthcare stakeholders, and fit-for-purpose scientific advice, so that developers would be able to gain clarity and invest in value-added medicines.
Recognition of value-added medicines as a separate category of medicines. Medicines for Europe’s Value Added Medicines Group is further recommending that value-added medicines be recognized as a separate category of innovation with incentives to encourage further development of value-added medicines in Europe. The group is proposing that, for the innovation on well-established substances, a noncumulative period of four years of data exclusivity be granted, subject to certain conditions and provided that the additional value that is delivered by the innovation can be demonstrated by appropriate data. It points out that data exclusivity as an incentive is already an established incentive in the pharmaceutical framework in the US and the introduction of a similar concept in Europe would bring the EU a step closer toward global convergence and set a level playing field for the EU’s pharmaceutical industry. It emphasizes that data exclusivity does not prevent competition from taking place as it does not translate into preventing other manufacturers from entering the market (as would be the case with market exclusivity).
Medicines for Europe’s Value Added Medicines Group says that the purpose of this proposal is to stimulate innovation where it is not currently encouraged and would not be used to further extend exclusivity for medicines that have already benefitted from an exclusivity period. “We, therefore, consider that the global marketing authorization (GMA) concept should continue to apply and that data protection would not apply to data that was previously used within the GMA (i.e., no further protection for studies already benefiting from exclusivity). The proposed framework should not facilitate the delay of the off-patent competition,” said Medicines for Europe’s Value Added Medicines Group in its white paper issued in November 2021. The group is calling for safeguards to achieve this balance.
Adoption of an evaluation framework for value-added medicines. Medicines for Europe’s Value Added Medicines Group is also calling for the adoption of an evaluation framework for value-added medicines in the reimbursement process. It says that the decision-making process should consider relevant value dimensions that demonstrate the benefits of value-added medicines in different purchasing /procurement mechanisms that should be defined with healthcare stakeholders: regulators, patients, healthcare professionals, and payers. It is recommending that pricing and reimbursement rules be shaped to adequately assess continuous innovation and be adjusted to the specificity of value-added medicines with different rules and a different assessment process than the current pathways for generic medicines (e.g., internal price referencing, mandatory discounts) or innovative medicines (e.g., clinical benefit) as it says that these are not appropriate for value-added medicines.
Rationale for change
Medicines for Europe’s Value Added Medicines Group is recommending these measures as it says that the current approaches to stimulate innovation of off-patent medicines have not been successful, the current approval process is too cumbersome, and the reimbursement process is not adequate. Further details are outlined below.
Current approval process is too cumbersome. The group further says the current approval process is too cumbersome and at present, there is no specific regulatory pathway for value-added medicines and that several legal based can apply for value-added medicines to be registered in the EU. “The consequence is high complexity for the applicant—different pathways require different levels of evidence and developers often need to seek regulatory advice to determine which legal pathway would be most suitable for their product,” said Medicines for Europe’s Value Added Medicines Group in its white paper. “This wastes resources and adds to the complexity and cost of the development. As continuous innovation is based on well-established substances, regulatory requirements should be further tailored to provide clarity for VAM [value-added medicine] developers by increasing predictability of required evidence and development costs and regulatory timelines.”
The absence of incentives for continuous innovation. Medicines for Europe’s Value Added Medicines Group further says that the current system does not encourage innovation for off-patent molecules and new measures are needed. “The current legislative framework encourages innovation in new chemical or biological entities and research for rare diseases or subsets of diseases. The EU pharmaceutical legislation also encourages follow-on competition at patent expiry with clear regulatory and market pathways for generic and biosimilar medicines, thereby massively increasing patient access to medicines,” said the group in its white paper. “However, the development of Value-Added Medicines requires additional evidence generation when compared to traditional follow-on products, with further complexity, costs, and prolongation of the development. Existing regulatory incentives in the EU framework, such as one year of non-cumulative data exclusivity for a new indication on a well-established substance have failed to stimulate more development—this pathway has never been used. Value-added medicines innovation is not sufficiently encouraged under either the generic, biosimilar or originator framework.”
Current reimbursement of value-added medicines. Medicines for Europe’s Value Added Medicines Group also says there is confusion in EU markets regarding the fair valuation of value-added medicine innovation. “The value assessment process is heterogeneous across EU Member States, and Value Added Medicines are often categorized as generic medicines because the innovation is on an off-patent molecule, with no process/framework in place to recognize their additional value in a proportionate way,” said the group in its white paper. “In other instances, VAM [value-added medicine] manufacturers are faced with requests for evidence in HTA [health technology assessment] processes that are designed for originator pharmaceuticals and, therefore, demand disproportionate evidence generation which is not fit for purpose and prevents affordable innovation from reaching patients.” By establishing an EU-level recognition of value-added medicines, Medicines for Europe’s Value Added Medicines Group says that EU member states can develop proportionate mechanisms to reward this kind of innovation.