White House Takes Next Steps To Onshore Mfg to US & Stockpiling APIs
FDA is seeking public comment through October 8 on how the US government can facilitate the onshoring of pharma manufacturing to the US. Also, President Donald Trump issues an Executive Order for stockpiling essential medicines in the US.
By Patricia Van Arnum, Editorial Director, DCAT, pvanarnum@dcat.org
Taking action to increase US domestic pharma manufacturing
The Trump Administration is taking several steps to facilitate the onshoring of pharma manufacturing into the US. Earlier this month (August 2025), the US Food and Drug Administration (FDA) announced the launch of FDA PreCheck, a new program designed to strengthen the domestic pharmaceutical supply chain by increasing regulatory predictability and facilitating the construction of manufacturing sites in the US. FDA PreCheck was developed in response to an Executive Order issued by President Donald Trump in May (May 2025) to streamline FDA review of pharmaceutical manufacturing facilities in the US, enhance the inspection process of foreign drug-manufacturing facilities, and streamline permitting of manufacturing facilities by the US Environmental Protection Agency.
In addition, next month (September 2025), FDA will host a public meeting, “Onshoring Manufacturing of Drugs and Biological Products” on September 30, 2025, at the FDA White Oak Campus in Silver Spring, Maryland, with virtual participation available. The meeting will feature a presentation of the FDA PreCheck draft framework, stakeholder discussions on framework strengths and opportunities, and discussions on additional considerations that may help overcome current challenges faced by industry to onshoring the manufacturing of pharmaceuticals, including active pharmaceutical ingredients (APIs) and finished drug and biological products, and ideas and options within the bounds of FDA’s statutory authority that could facilitate such onshoring of manufacturing.
At the same time, FDA is establishing a docket to solicit public comments on the FDA PreCheck program proposal and other issues related to accelerating the establishment of new pharmaceutical manufacturing facilities in the US. Figure 1 outlines the topics in which FDA is seeking public input. The public comment period ends October 8, 2025.
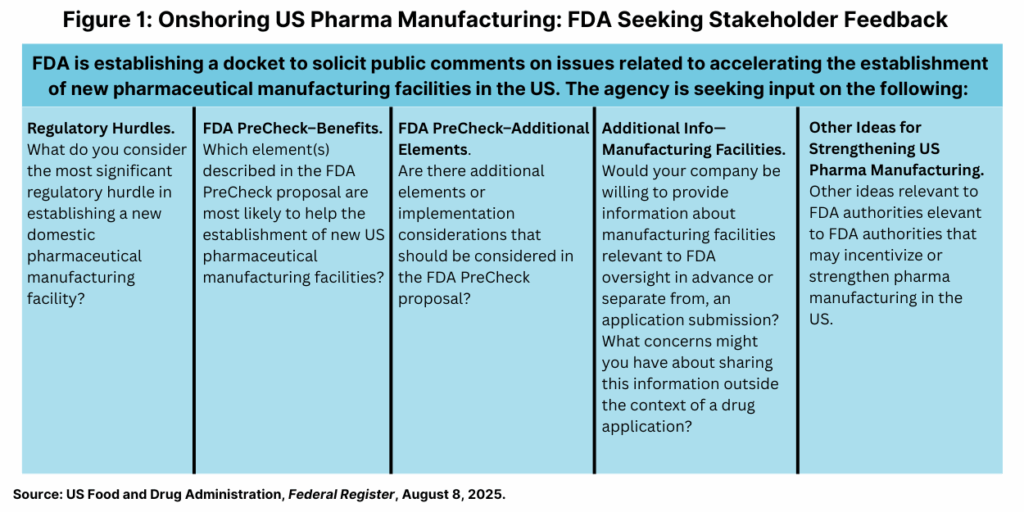
The impetus for these moves is to address an imbalance between domestic and foreign manufacturing of pharmaceuticals marketed in the US and to increase US-based domestic manfacturing. According to information from FDA, as of June 2025, approximately 53% of brand drug products and 69% of generic drug products have at least one manufacturer outside of the United States. Additionally, as of 2025, 9% of API (Type II) Drug Master File (DMF) holders are in the United States, 22% are in China, and 44% are in India.
FDA’s PreCheck program proposal
FDA’s PreCheck aims to support faster establishment of new US pharmaceutical manufacturing capacity through earlier regulatory input, enhanced engagement, and efficient chemistry, manufacturing and controls (CMC) assessments. Specifically, the proposal consists of a two-phase approach: (1) the Facility Readiness Phase and (2) the Application Submission Phase.
Phase 1: Facility Readiness Phase
The first phase of the program, the Facility Readiness Phase, is intended to help enable early facility engagement and support through pre-operational review and to allow manufacturing facility information provided via a Type V DMF. The pre-operational review consists of two main components as outlined below.
- Enables manufacturers to seek FDA feedback, as applicable, at critical facility development stages, including facility design, pre-construction, construction/equipment installation and qualification, and pre-production phases.
- Provides manufacturers with insight into whether their planned facility and manufacturing operations as designed are likely to comply with cGMP requirements.
In addition, under the Facility Readiness Phase, companies will be able to provide FDA with manufacturing facility information provided via a Type V DMF. The types of information and the benefits of being able to supply this information are outlined below.
- Offers industry an opportunity to provide FDA with a comprehensive master file that contains facility-specific information including, for example, site operations layout and description found in a Site Master File, Pharmaceutical Quality System elements, and Quality Management Maturity practices.
- Helps FDA to provide timely feedback on consistency and effectiveness of quality procedures to reduce the risk of cGMP deficiencies that could compromise product quality, patient safety, and application approval.
- Serves as a living document that is updated throughout the facility lifecycle that, as appropriate, can be incorporated by reference into a drug application, and can be leveraged to streamline facility assessments during application reviews.
Phase 2: Application Submission Phase
The Application Submission Phase of the FDA PreCheck program is intended to help facilitate enhanced and accelerated quality assessment through pre-application meetings and engagements. The benefits to the applicant are outlined below.
- Provide applicants and their manufacturers the opportunity to give FDA advanced awareness of facility and manufacturing strategies for specific drugs in forthcoming applications while enabling earlier assessment and inspection activities within the review cycle.
- Enable FDA to provide CMC feedback on anticipated data or logistical needs to support timely review and inspection processes.
- Allow FDA to accelerate quality element assessments for applications from new U.S. facilities through early facility engagement and frontloaded assessment activities.
Increasing the US national stockpile of APIs of essential medicines
The Trump Administration made another move this week (August 13, 2025) to US-based manufacturing by issuing an Executive Order to increase the national stockpile for active pharmaceutical ingredients (APIs) of essential medicines. This follows earlier actions under the first Trump Administration. In 2020, the President issued an Executive Order to increase the domestic procurement of essential medicines, medical countermeasures, and critical inputs and to identify supply chain vulnerabilities. Following up on that order, FDA published a list of essential medicines, medical countermeasures, and critical inputs in October 2020, which the Office of the Assistant Secretary for Preparedness and Response within the Department of Health and Human Services later reduced to 86 essential medicines. Also, at that time, the Trump Administration created a Strategic Active Pharmaceutical Ingredients Reserve (SAPIR) to stockpile APIs. The SAPIR was seen as a way to insulate the US from the concentration of foreign supply of key starting materials and APIs and to encourage more domestic production of APIs through government purchases of APIs.
The new Executive Order issued this week (August 13, 2025) builds on that framework with several key actions to increase the US national stockpile of APIs for essential medicines as outlined below.
- Directs the Office of the Assistant Secretary for Preparedness and Response (ASPR), within the Department of Health and Human Services, to develop a list of approximately 26 critical drugs vital to US national health and security and ready the SAPIR repository to receive and maintain the APIs used to make these critical drugs.
- Charges the ASPR with obtaining a six-month supply of these APIs, with a preference for obtaining domestically manufactured APIs if possible, and placing them in the SAPIR.
- Further instructs the ASPR to update the 2022 list of 86 essential medicines and propose a plan to obtain and store a six-month API supply for these drugs.
- Directs ASPR to further develop a proposal for opening a second SAPIR repository to further enhance pharmaceutical supply chain resilience.
The Trump Administration says these actions to increase the US national stockpile of APIs for essential medicines is required to specifically address shortfalls in US domestic API production. A fact sheet accompanying the Executive order says that only about 10% of the APIs by volume for the finished drug products used in the US are made in the US.







