Demonstration of Equivalency Between Two Analytical Methods for the Determination of Loss on Drying (LOD) in Empty Hard Gelatin and Hypromellose Capsules
1.Introduction
Moisture in empty gelatin and Hypromellose (HPMC) capsules are tested and controlled within certain limits to ensure quality, shelf-life, and functionality. While the United States and the European Pharmacopoeia do not have official monographs for empty gelatin and HPMC capsules, the US Pharmacopeia has proposed monographs for these two capsule types, including specifications for moisture content1. Moisture content of the empty capsules is determined by loss on drying (LOD) and the proposed USP monographs for empty gelatin and HPMC capsules use a drying oven (DO) method for the determination of moisture content. Most dietary supplement and pharmaceutical manufacturers use the DO method to test for the moisture content of incoming shipments of capsules.
One major disadvantage of the DO method is that it is slow, requiring at least four hours to complete moisture testing of one sample. The DO method is not suitable for use as an in-process quality control test for moisture content during production of empty capsules as it takes too long to complete and requires qualified laboratory analysts. The Halogen moisture Analyser (HMA) method is a faster alternative to the DO method; measuring moisture content with this method can be completed within 15 minutes and therefore it is a preferred method for in-process testing of moisture content in empty capsules. An empty capsule manufacturer using the moisture content data generated by the HMA method for the release of capsules should demonstrate the results for moisture content generated by the HMA and DO methods are equivalent since the use of non-equivalent methods may result in out-of-specification results when the customer tests the capsules.
In a method equivalency study, multiple samples are tested, and each sample is tested by the test and reference methods. The paired data is subjected to statistical analyses to determine a 95% confidence interval (a range of values that we can be 95% confident contains the true mean difference) for equivalence of test and reference means. The paired sample design provides a way to control for the sample-to-sample variation. The 95% confidence interval is compared to an equivalence interval. For the demonstration of equivalency of two methods used to measure the moisture content of empty capsules, ±5% of the mean LOD for the reference method (DO method) can be considered as the equivalence interval. If the confidence interval is contained within the equivalence interval, the two methods are considered equivalent.
2.0 LOD method equivalency study for empty gelatin and HPMC capsules
2.1 Sample preparation and testing
2.1.1 Gelatin Capsules
For the method equivalency study with empty gelatin capsules, 10 samples of capsules were used. Two sub-samples were prepared from each of these samples and tested for LOD using the DO and the HMA methods. For the DO method, approximately 1.0 to 2.0 grams of capsules were weighed at time 0 and after 4 hours of drying at 105°C. For the HMA method approximately 2.0 grams of capsules were heated to 140°C for 6 minutes.
2.1.2 HPMC capsules
Method equivalency study with HPMC capsules was performed with sub-samples prepared from eight capsule samples, which were tested for LOD using the two methods. For the DO method, approximately 1.0 to 2.0 grams of capsule samples were weighed at time 0 and after 4 hours of drying at 105°C. For the HMA method, approximately 2 grams of capsules were heated to 140°C for 3 minutes.
2.2 Results for the gelatin capsules
Table 1 shows the results for the LOD for the ten capsule samples determined by the DO and the HMA methods. Means for the LODs obtained for the DO and HMA methods are 14.2% and 14.3%, respectively.
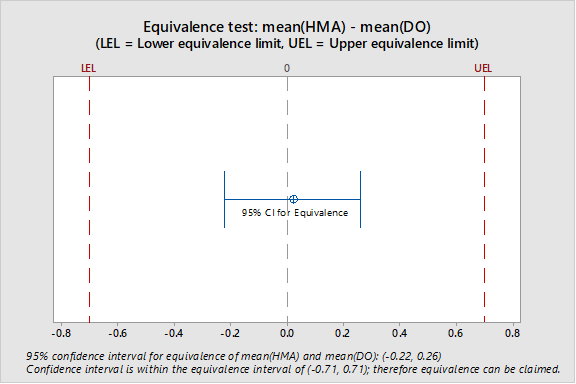
Mean LOD for the DO method (14.2%) ±5% was used as the equivalence interval. Figure 1 shows the location of the 95% confidence interval in relation to the equivalence interval (-0.71 to +0.71); the 95% confidence interval is contained within the equivalence interval.
Table 1: LOD Results Generated by the DO and the HMA Methods
| Sample # | Oven method % LOD data for sub- sample A | HMA method % LOD data for sub-sample B | Sub-Sample B – A |
| 1 | 13.2 | 13.5 | 0.3 |
| 2 | 13.6 | 13.9 | 0.3 |
| 3 | 14.5 | 14.7 | 0.2 |
| 4 | 15.1 | 14.3 | -0.8 |
| 5 | 14.9 | 15.2 | 0.3 |
| 6 | 14.6 | 14.7 | 0.1 |
| 7 | 14.8 | 14.5 | -0.3 |
| 8 | 14.2 | 13.7 | -0.5 |
| 9 | 13.2 | 13.3 | 0.1 |
| 10 | 14.2 | 14.7 | -0.5 |
Figure 1: Location of Confidence Interval for the Difference between the Two Mean LODs in Relation to Equivalence Interval
2.3 Results for the HPMC Capsules
Table 2 below shows the results for the LOD for the eight capsule samples determined by the DO and the HMA methods. Means for LODs obtained for both the DO and HMA methods are 5.3%.
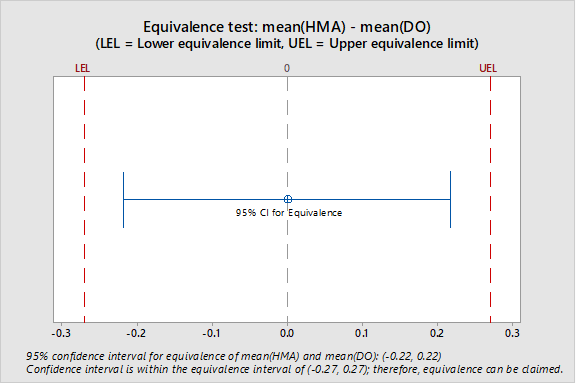
Mean LOD for the DO method (5.3%) ±5% was used as the equivalence interval. Figure 2 shows the location of the 95% confidence interval in relation to the equivalence interval (-0.27 to +0.27); the 95% confidence interval is contained within the equivalence interval.
Table 2: LOD Results Generated by the DO and the HMA Methods
| Sample # | Oven method % LOD data for sub-sample A | HMA method % LOD data for sub-sample B | Sub-sample B – A |
| 1 | 6.9 | 6.3 | -0.6 |
| 2 | 6.4 | 6.2 | -0.2 |
| 3 | 6.2 | 6.5 | 0.3 |
| 4 | 5.5 | 5.7 | 0.2 |
| 5 | 5.8 | 5.6 | -0.2 |
| 6 | 3.7 | 4.1 | 0.4 |
| 7 | 3.9 | 4.0 | 0.1 |
| 8 | 3.9 | 3.9 | 0 |
Figure 2: Location of Confidence Interval for the Difference between the Two Mean LODs in Relation to Equivalence Interval
3.0 Conclusions
For both the empty gelatin and the HPMC capsules, since the 95% confidence interval for the difference between the two means falls within the equivalence interval, it is concluded that the HMA and the DO methods used for the determination of moisture content in these two capsules are equivalent. A change to the temperature and or the time used for the HMA method would require a revaluation of method equivalence.
4.0 References
- Pharmaceutical Forum. November 2022; 48 (6).



