COVID-19 Vaccines: Manufacturing and Supply
Three COVID-19 vaccines by Pfizer/BioNTech, Moderna, and J&J have been authorized for emergency use in the US. What are the supply targets for 2021 and beyond, and what manufacturing networks (internal and external) are being used? DCAT Value Chain Insights examines the latest.
Vaccine technologies
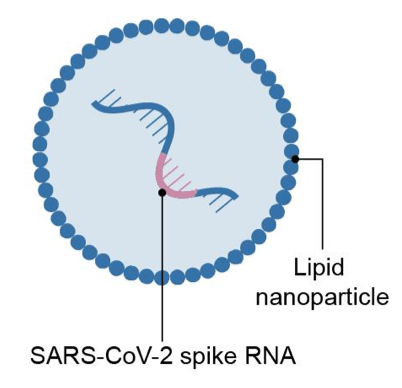
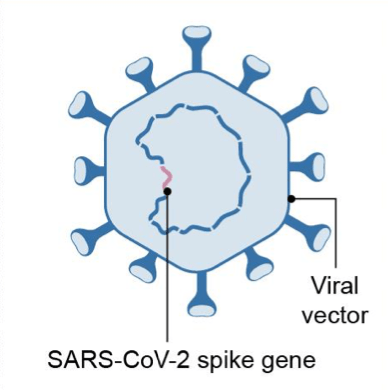
The COVID-19 vaccines that have received emergency use authorization from the US Food and Drug Administration (FDA) to date (as reported on March 8, 2021) respectively from Pfizer/BioNTech, Moderna, and Johnson & Johnson (J&J) are based on different vaccine technologies. The Pfizer/BioNTech and Moderna vaccines are based on messenger RNA (mRNA) vaccine platforms. In a mRNA vaccine, encapsulated genetic instructions allow vaccinated individuals to produce the spike protein of SARS-CoV-2, the virus that causes COVID-19, to stimulate the immune system. J&J’s COVID-19 vaccine is manufactured using a specific type of virus called adenovirus type 26 (Ad26). The vaccine uses Ad26 to deliver a piece of the DNA, or genetic material, that is used to make the distinctive “spike” protein of the SARS-CoV-2 virus, the virus that causes COVID-19. J&J’s COVID-19 vaccine uses its AdVac vaccine platform, a proprietary technology that was also used to develop and manufacture Janssen’s Ebola vaccine regimen (which was approved by the European Commission) and construct its investigational Zika, respiratory syncytial virus, and HIV vaccines. Table I summarizes six COVID-19 vaccines that the US government has supported through funding either for development and/or supply agreements. The material is based on a recent report by the US Government Accountability office.
| Table I: Vaccine Platform Technologies Supported by US Government/Operation Warp Speed | ||||
| Vaccine Platform | mRNA platform | Replication-defective live vector platform | Recombinant-subunit adjuvanted protein platform | Attenuated replicating live-vector platform |
 |
 |
 |
 |
|
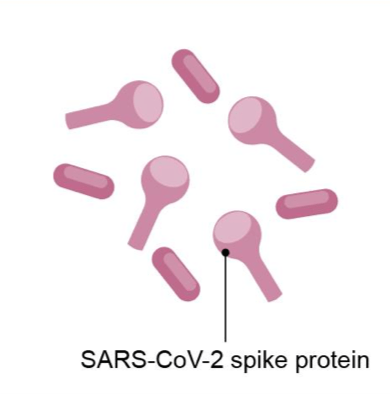
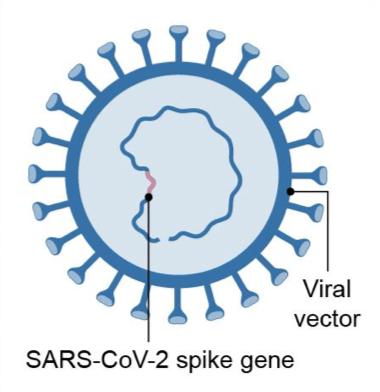
| Description | Encapsulated genetic instructions that allow vaccinated individuals to produce the spike protein of SARS-CoV-2 to stimulate immune system but cannot cause COVID19 | Non-replicating virus that delivers genetic instructions to allow vaccinated individuals to produce the spike protein of SARS-CoV-2 to stimulate immune system but cannot cause COVID-19 | Fully-formed spike protein of SARS-CoV-2 delivered with adjuvant, which helps to stimulate immune system of vaccinated individuals but cannot cause COVID-19 | Replicating virus that delivers genetic instructions to allow vaccinated individuals to produce the spike protein of SARS-CoV-2 to stimulate immune system but cannot cause COVID-19 |
| Companies working with US government for vaccine |
Pfizer/BioNTech Moderna |
Johnson & Johnson’s Janssen AstraZeneca |
Novavax Sanofi/GlaxoSmithKline |
none |
| Technology used in prior FDA licensed vaccines | No vaccine using this technology has ever been licensed by FDA | No vaccine using this technology has ever been licensed by FDA* | Example: seasonal influenza vaccine (licensed by FDA) | Example: Ebola vaccine (licensed by FDA) |
| Authorized for emergency use in the US (as of March 8, 2021) |
Pfizer/BioNTech Moderna |
Johnson & Johnson’s Janssen | ||
| Phase of development for FDA full licensure |
Pfizer/BioNTech (Phase III) Moderna (Phase III) |
Johnson & Johnson’s Janssen (Phase III) AstraZeneca (Phase III) |
Sanofi/GSK (Phase II) Novavax (Phase III) |
none |
|
Note: For vaccines that received emergency use authorization (EUA) from FDA, additional data on vaccine effectiveness will be generated from further follow-up of participants in clinical trials already underway before the EUA was issued. *Note: J&J’s COVID-19 vaccine uses its AdVac vaccine platform, a proprietary technology that was also used to develop and manufacture Janssen’s Ebola vaccine regimen (which was approved by the European Commission) and construct its investigational Zika, respiratory syncytial virus, and HIV vaccines. Source: US Government Accountability Office (GAO) report, Operation Warp Speed: Accelerated COVID19 Vaccine Development Status and Efforts to Address Manufacturing Challenges (February 2021) and company information. Per the GAO report, adaption of images from the GAO report depicting various vaccine technologies were with permission from Springer Nature:(Nature, “The Race for Coronavirus Vaccines: A Graphical Guide,” Ewen Calloway ©2020, GAO 21-319. |
||||
Developing and gaining authorization for a COVID-19 vaccine is only one challenge in delivering the vaccine. Equally as challenging is ramping up large-scale production, through a combination of internal manufacturing capacity and partnered external manufacturing capacity. Below are highlights of Pfizer/BioNTech’s, Moderna’s and J&J manufacturing capacity.
Manufacturing and supply: Pfizer/BioNTech
As reported in late February (February 2021), Pfizer/BioNTech is on track to make 120 million doses of their vaccine available for shipment by the end of March (March 2021) and an additional 80 million doses by the end of May (May 2021). Pfizer anticipates all 300 million contracted doses with the US government will be made available for shipment by the end of July (July 2021), enabling the vaccination of up to 150 million individuals in the US. Last month (February 2021), the US government purchased an additional 100 million doses of the COVID-19 vaccine from Pfizer and BioNTech to bring the total amount of doses purchased by the US government to 300 million.
Pfizer’s facility in Kalamazoo, Michigan remains the primary manufacturing site of the vaccine in the US. The company has also made investments at its other US manufacturing sites, including Saint Louis, Missouri; Andover, Massachusetts; and Pleasant Prairie, Wisconsin. In addition, the company added new lines at its site in McPherson, Kansas, started lipid production at its site in Groton, Connecticut, and added contract manufacturers.
Pfizer and BioNTech also formed recent agreements for the supply of lipids for the vaccine. In late February (February 2021), AMRI was chosen to support the development and manufacture of lipid excipients for the Pfizer-BioNTech COVID-19 vaccine. For the past several months (as reported on February 25, 2021), AMRI has been providing development, scale-up and manufacturing services at a number of its global R&D and production sites to deliver capacity for the lipid excipient supply for the vaccine. Also in February (February 2021), Evonik, a specialty and fine chemicals producer, announced it is expanding its specialty lipids produced at its Hanau and Dossenheim sites in Germany as part of a strategic partnership with BioNTech. BioNTech is also expanding its strategic partnership with Merck KGaA to accelerate the supply of lipids and increase the amount of lipid delivery toward the end of 2021.
Pfizer’s and BioNTech’s manufacturing network outside the US includes BioNTech’s facility in Marburg, Germany and external partners. In late January (January 2021), Novartis signed an initial agreement to use its manufacturing capacity and capabilities for the production of the Pfizer/BioNTech vaccine. Under the terms of the contract manufacturing agreement, Novartis plans to take bulk mRNA active ingredient from BioNTech and fill this into vials under aseptic conditions for shipment back to BioNTech for BioNTech’s distribution. Subject to reaching a final agreement, Novartis plans to begin production in the second quarter of 2021 at its aseptic manufacturing plant in Stein, Switzerland. Initial shipment of finished product is expected in the third quarter of 2021. Pfizer/BioNTech are also partnered with Sanofi for their COVID-19 vaccine. As part of a pact announced in January (January 2021), from the summer of 2021, Sanofi will perform late-stage manufacturing to supply over 125 million doses of the vaccine for the European Union.
Globally, as reported in mid-February (February 2021), Pfizer/BioNTech plan to have increased manufacturing capacity to up to 2 billion doses for 2021, assuming continuous process improvements, expansion at current facilities, adding new suppliers and contract manufacturers, and an updated six-dose labeling (after dilution, vials of Pfizer/BioNTech’s COVID-19 vaccine contains six doses of 0.3-mL of vaccine).
Manufacturing and supply: Moderna
Moderna is on track to meet its commitment to deliver 100 million doses of its COVID-19 vaccine to the US government by the end of March (March 2021). The company reported in late February (February 2021) that it is aiming to double its deliveries by April (April 2021) to more than 40 million doses per month. The US government purchased an additional 100 million doses of Moderna’s COVID-19 vaccine last month (February 2021) to bring the total amount of doses purchased by the US government to 300 million. Moderna says it is aiming to deliver a second hundred million doses by the end of May (May 2021) and a third hundred million doses by the end of July (July 2021).
Moderna reported in late February (February 2021), it is making new capital investments to increase capacity at its owned and partnered manufacturing facilities, which it expects will increase global 2022 capacity to approximately 1.4 billion doses of its COVID-19 vaccine, assuming a 100-μg dose. The investments will enable additional production of Moderna’s current COVID-19 vaccine and provide flexibility in addressing production of potential vaccine boosters that may be needed to address emerging variants of SARS-CoV-2, the virus that causes COVID-19.
The 2022 capacity of up to 1.4 billion doses reflects an assumption of a 100-μg dose. The total 2022 supply will depend on the mix between the authorized COVID-19 vaccine at 100 μg and the dose level authorized for a booster. In the event that the company dedicates its entire 2022 capacity to a 50-μg boost, Moderna could supply up to 2.8 billion doses in fiscal year 2022. The maximum output will be determined as the company more fully develops its booster product strategy.
The company has already begun adding this capacity at its owned and partnered manufacturing facilities. Given a six- to nine-month timeframe to add capacity and an additional timeframe to permit regulatory validation and ramp-up, it is estimated that up to 12 months may be necessary before the additional production is available.
Also, in late February (February 2021), Moderna announced it is increasing its base plan for 2021 manufacturing from 600 million doses to 700 million doses globally. Moderna said it is exploring other approaches to potentially improve throughput and is working to further optimize its operations to potentially deliver up to 1 billion doses in fiscal year 2021.
Moderna is partnered with several external partners in the US and outside the US, including Baxter BioPharma Solutions, Lonza, and Rovi. Earlier this month (March 2021), Moderna partnered with Baxter BioPharma Solutions for Baxter to provide fill–finish sterile manufacturing services and supply packaging for approximately 60 million to 90 million doses of Moderna’s COVID-19 vaccine in 2021 at Baxter’s facility in Bloomington, Indiana.
Manufacturing and supply: J&J
Earlier this month (March 2021), the US government announced that it will collaborate with Merck & Co. to repurpose some of existing Merck facilities for rapid large-scale manufacturing of vaccines and therapeutics for use in public health emergencies, including the current pandemic. The facilities will be available to private sector partners working with the US federal government on the COVID-19 response or to produce Merck products against COVID-19. J&J’s Janssen Pharmaceuticals will be the first federal partner to use repurposed Merck facilities to manufacture a COVID-19 vaccine. Merck will use two of its facilities in the US to produce drug substance, formulate, and fill vials of the vaccine, according to information from the US Department of Health and Human Services (HHS).
The Biomedical Advanced Research and Development Authority, part of the HHS Office of the Assistant Secretary for Preparedness and Response, will provide up to $268.8 million in funding to Merck, with an initial investment of $105 million, to adapt and make available a number of existing manufacturing facilities for the production of SARS-CoV-2/COVID-19 vaccines and medicines. This funding is in addition to Merck’s continued investment in its global vaccines manufacturing network as part of its planned capital investments of more than $20 billion from 2020 through the end of 2024.
To further accelerate production of the J&J vaccine, President Joe Biden further announced he has invoked the Defense Production Act to expedite materials in vaccine production, such as equipment, machinery, and supplies, such as single-use bags. He has directed the Department of Defense to provide daily logistical support to strengthen J&J’s efforts. J&J also will begin operating its manufacturing facilities 24/7 to maximize production output.
These efforts will contribute to J&J’s ability to accelerate delivery of its vaccine doses from 100 million doses by the end of June (June 2021) to at or near 100 million doses by the end of May (May 2021), according to the HHS. In the long term, the HHS says these actions will ultimately double J&J’s US capacity to produce drug substance and increase US capacity for fill-finish.
Following the announcement of Merck’s participation to manufacture J&J’s vaccine, the US government announced on March 10, 2021, it will purchase an additional 100 million doses of J&J’s COVID-19 vaccine. The agreement by the US government to purchase an additional 100 million doses of the vaccine follows an agreement between the US government and J&J signed last August (August 2020) for an initial 100 million doses of the vaccine.
J&J says it expects this manufacturing arrangement will enhance production capacity so that the company can supply beyond its current commitments. Merck is the ninth manufacturer to join J&J’s global network for manufacturing the COVID-19 vaccine. J&J also formed an agreement with Sanofi in which Sanofi will provide manufacturing support for J&J’s COVID-19 vaccine.
Editor’s Note: This article was updated to include the US government’s decision on March 10, 2021 to purchase an additional 100 million doses of J&J’s COVID-19 vaccine.