Raising the Bar: The Innovation Leaders
Innovation is central to the bio/pharma industry, so what have been the key developments in 2025? Which companies and their innovation stand out? DCAT Value Chain Insights takes an inside look.
By Patricia Van Arnum, Editorial Director, DCAT, pvanarnum@dcat.org
AI partnership
Artificial intelligence (AI) applied in drug discovery and development is of high interest, and a noteworthy move in 2025 is a partnership forged between Eli Lilly and Company and Nvidia, the high-tech giant and maker of computer chips used in AI. In October (October 2025), Lilly announced it is building a supercomputer for drug-discovery and development, in collaboration with Nvidia, which produces graphics processing units (GPUs), systems on chips, and application programming interfaces (APIs) for data science, high-performance/accelerated computing and AI. The supercomputer will power an “AI factory,” a specialized computing infrastructure that manages the entire AI lifecycle from data ingestion and training to fine-tuning and high-volume inference. The supercomputer will be the world’s first Nvidia DGX SuperPOD with DGX B300 systems, according to information from Lilly. It will be powered by more than 1,000 B300 GPUs on a unified networking fabric, which means communication across GPUs, storage and related systems runs on one high-speed network.
The new supercomputer and AI factory is designed to enable rapid learning and iteration. Scientists will be able to train AI models on millions of experiments to test potential medicines, thereby expanding the scope and sophistication of drug-discovery efforts. A number of these proprietary AI models will be available on Lilly TuneLab, a collaborative federated AI/machine-learning drug-discovery platform created to expand access to advanced discovery tools across the biopharma ecosystem.
Beyond discovery, Lilly plans to use the supercomputer to shorten development cycles. New scientific AI agents can support researchers in reasoning, planning and collaborating across digital and physical environments. With advanced medical imaging, scientists would benefit from a clearer view of how diseases progress and can develop new biomarkers for more personalized care. Manufacturing processes can benefit from digital twins together with Nvidia’s robotic technologies to improve production efficiency and reduce downtime.
Advanced manufacturing:
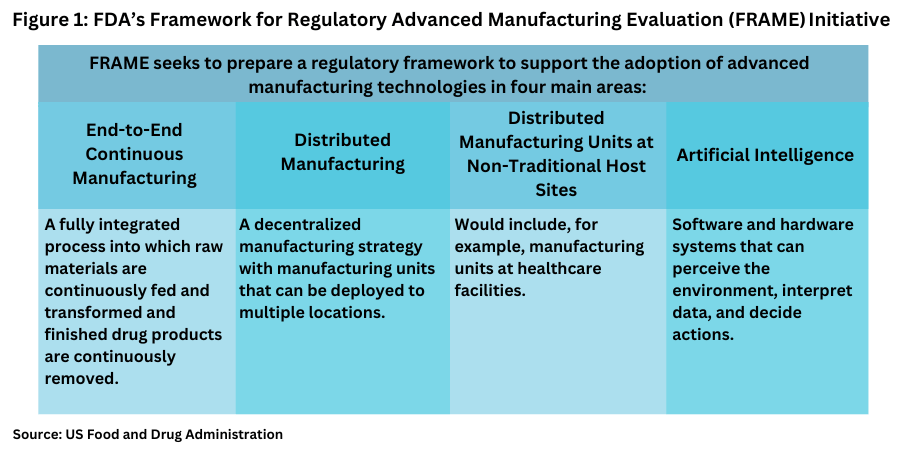
In looking at innovation in the bio/pharma industry, product innovation is front and center, but innovation in manufacturing is also an important area and ways to cultivate that innovation are also important, Adopting innovative approaches to manufacturing may present both technical and regulatory challenges. On the regulatory front, pharmaceutical companies may have concerns that developing technologies could result in delays while novel regulatory challenges are considered. This is especially true while regulatory assessors familiarize themselves with new technologies and determine how they may be evaluated within the existing regulatory framework. To address these concerns, the Office of Pharmaceutical Quality under the Center for Drug Evaluation and Research (CDER) under the US Food and Drug Administration created the Emerging Technology Program in 2014 as a collaborative program where industry representatives can meet with Emerging Technology Team members to discuss, identify, and resolve potential technical and regulatory issues regarding the development and implementation of a novel technology prior to filing a regulatory submission. CDER established the Framework for Regulatory Advanced Manufacturing Evaluation (FRAME) initiative to prepare a regulatory framework to support the adoption of advanced manufacturing technologies. The FRAME initiative prioritized four technologies (see Figure 1), and several interesting projects in 2025 were announced.

Advanced manufacturing projects
Advanced manufacturing is seen as a way to make manufacturing more cost-efficient and competitive, and in May (May 2025), the US government launched four projects aimed at advancing pharmaceutical manufacturing in the US using AI, machine learning, and informatics for certain generic drugs. The program, called Equip-A-Pharma, is a collaboration between the US Department of Health and Human Services (HHS), the Administration for Strategic Preparedness and Response (ASPR), the Defense Advanced Research Projects Agency (DARPA), and the private sector. Advanced manufacturing is seen as a way to make manufacturing more cost-efficient and competitive.
Over the next year (as reported on May 15, 2025), each company and its partners will aim to show how their technologies can potentially make active pharmaceutical ingredients and specific finished drug formulations at the point-of-care. All of the partners in these projects are expected to submit abbreviated new drug applications to the US Food and Drug Administration within a year (as reported on May 15, 2025) as a result of leveraging the government-supported development work.
“Manufacturing medicines from start to finish in the United States ensures a secure supply of essential medications,” said HHS Principal Deputy Assistant Secretary for Preparedness and Response John Knox, in a May 15, 2025, statement in commenting on the launch of the program. “Traditional pharmaceutical manufacturing is often too rigid and slow to adapt to changing demands, especially during national emergencies. We’re launching projects aimed at completely changing the approach not just to bring pharmaceutical manufacturing back to the US but to do it better.”
By producing active pharmaceutical ingredients and finished medications at the point of care, such as a hospital, these types of technologies can strengthen domestic supply chains, ASPR says, and that this approach could mitigate drug shortages, enhance national health security and improve resilience. These projects address US-based manufacturing of eight sterile injectable or oral medications and the active pharmaceutical ingredients for these medicines. The partners conducting the projects are Battelle Memorial Institute and Aprecia; BrightPath Laboratories; Rutgers University; and the Mark Cuban Cost Plus Drug Company.
Partners Battelle Memorial Institute and Aprecia are integrating advanced process analytics and machine learning that allows for real-time quality assessment and supports a digital approach to generating regulatory submissions. The companies are using their own technologies to produce 3-D printed oral solid dosage forms (tablets) of levetiracetam to treat epilepsy or other seizures, and linezolid to treat gram-positive infections such as bacterial pneumonia.
Bright Path is demonstrating technology for continuous flow manufacturing to produce lidocaine HCl, a local anesthetic, and carboplatin, a chemotherapy drug. The project is expected to show enhanced efficiency, scalability, adaptability of the technology, rapid reconfiguration between products with minimal downtime, and a real-time digital approach to generating regulatory submissions. The technology integrates advanced process informatics and in-process metrology.
Rutgers University will demonstrate manufacturing of registration batches for two drugs, bupivacaine HCl, an anesthetic, and albuterol sulfate, used to treat asthma, bronchitis, emphysema or other lung conditions. Rutgers has been working with ASPR’s Center for Industrial Base Management and Supply Chain and DARPA to develop a manufacturing platform and generate data sets using new AI tools that can inform future regulatory submissions and rapid release of products.
The Mark Cuban Cost Plus Drug Company is employing a highly automated and flexible manufacturing platform that integrates AI and machine learning for real-time quality assessment and process optimization. The company is demonstrating use of the platform to process active pharmaceutical ingredients and the finished medication doses rapidly with in-line metrology to produce lidocaine, a widely used anesthetic, and diltiazem, a calcium channel blocker used to treat heart conditions. The system uses AI-driven informatics, is scalable and adaptable, and can be used by operators with minimal training to maintain product quality.
Other industry projects
In January (January 2025), Purdue University, in collaboration with Eli Lilly and Company and Merck & Co., announced the launch of the Young Institute Pharmaceutical Manufacturing Consortium, a collaboration with a focus on sterile injectables and innovative aseptic manufacturing technology. Elizabeth Topp, Director of the Young Institute, will serve as the new consortium’s Director. She is a professor in the Department of Industrial and Molecular Pharmaceutics and the Davidson School of Chemical Engineering at Purdue University with expertise in improving the shelf life and stability of pharmaceuticals.

The members of the new consortium say they anticipate the consortium will attract broad participation throughout the sector from other pharmaceutical companies, pharma contract manufacturers, equipment manufacturers, start-ups, and venture capital firms. The consortium will look to create systems equipped with automated visual inspection and in-line process and product-quality monitoring, all of which will require a new generation of skilled pharmaceutical manufacturing engineers and scientists. The consortium will seek to advance manufacturing by developing innovative technologies, autonomous systems, smart AI, and related digital technologies, together with industrially relevant education and training. The collaboration also underscores a commitment to onshoring pharmaceutical manufacturing while bolstering domestic production.
In addition, in May (May 2025), Purdue University and a coalition of leaders in AI, pharmaceutical manufacturing, and public policy launched a national effort to onshore pharmaceutical manufacturing in the US by applying AI and advanced manufacturing technologies. Led by Purdue, the Information Technology and Innovation Foundation, and the National Institute for Pharmaceutical Technology and Education, coalition representatives signed a collaborative accord that outlined concrete objectives to lower production costs, improve quality control, build a geographically distributed manufacturing base, accelerate regulatory pathways, and train a new generation of skilled talent to lead in AI-enabled pharmaceutical innovation as a means to strengthen US-based pharmaceutical manufacturing. Other signatories to the accord included Eli Lilly and Company, Evonik, Phlow Corporation, Google, and Blackstone Life Sciences, among others.