Top 10 Watch List: Pharma CEOs on the Rise
The quest for vaccines and treatments against COVID-19 has put the CEOs of certain companies, both large and small, on the industry’s radar. The CEOs of J&J, AstraZeneca, Pfizer, Moderna, and Regeneron Pharmaceuticals are some executives to watch in 2020, but who else is making the mark?
Pharma CEOs to watch in 2020
In any given year, the chief executive officers (CEOs) on the large biopharmaceutical/pharmaceutical companies would make a list of the top executives for their strategies, growth prospects, and deal-making. Although those elements factor into our decision-making in compiling the DCAT Value Chain Insights’ Watch List: CEOs on the Rise for 2020 (see Figure 1), additional criteria factor into this year’s selection: the success or not of developing a vaccine or potential treatments against the novel coronavirus (COVID-19). With the pharma industry focused front and center on developing COVID-19 vaccines and treatments, we narrowed the list to those companies, both large and smaller, with advanced COVID-19 candidates as well as smaller companies making important drug-development partnering deals outside of COVID-19 projects. The Top 10 list is provided below.
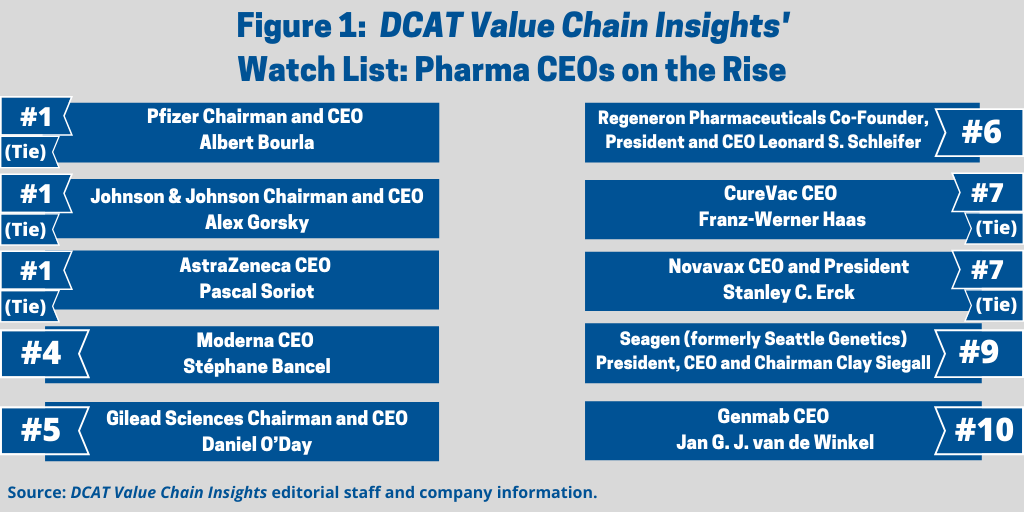
1. Three-way tie: Pfizer, J&J, and AstraZeneca. At the top of the list, and tied for first place, are the CEOs of three pharmaceutical majors—Pfizer, Johnson & Johnson (J&J), and AstraZeneca—with late-stage COVID-19 vaccine candidates and significant investments to produce and supply those vaccines.
Pfizer Chairman and CEO Albert Bourla. Pfizer Chairman and CEO Albert Bourla reported last week (October 16, 2020) that the company’s lead COVID-19 vaccine candidate, BNT162b2, which it is developing with BioNTech, a Mainz, Germany-based immunotherapy company, may be ready for submission for emergency use authorization (EUA) to the US Food and Drug Administration (FDA) in the third week of November (November 2020). For the EUA, the FDA is requiring that companies provide two months of safety data on half of the trial participants following the final dose of the vaccine. Based on the company’s current trial enrollment and dosing pace, Pfizer estimates that it will reach this milestone in the third week of November.
BNT162b2 is based on BioNTech’s proprietary messenger ribonucleic acid (mRNA) technology and is supported by Pfizer’s global vaccine development and manufacturing capabilities. The vaccine candidate is currently being evaluated in an ongoing global Phase III study at more than 120 clinical sites worldwide, including the US, Brazil, South Africa, and Argentina. The companies reported earlier this year (July 2020) that if authorization or approval is obtained, the companies currently aim to supply globally up to 100 million doses by the end of 2020 and approximately 1.3 billion doses by the end of 2021.
J&J Chairman and CEO Alex Gorsky. J&J Chairman and CEO Alex Gorsky has also allocated significant resources to develop and manufacture the company’s COVID-19 vaccine candidate. The investigational COVID-19 vaccine uses the company’s AdVac technology, which uses an adenovirus as a vector (a carrier), which has been genetically modified so that it can no longer replicate in humans and cause disease. The technology was also used to develop and manufacture the company’s Ebola vaccine, which was approved by the European Commission earlier this year (2020) and for the constructs of its vaccine candidates respectively for Zika, respiratory syncytial virus, and HIV.
Earlier this month (October 2020), the company temporarily paused all dosing in its COVID-19 vaccine candidate trials, including its Phase III Ensemble trials, due to an unexplained illness in a study participant. The company says that the participant’s illness is being reviewed and evaluated by an independent data safety monitoring board as well by the company’s internal clinical and safety physicians. The company noted that a study pause, in which recruitment or dosing is paused by the study sponsor, is a standard component of a clinical trial protocol. J&J initiated its Ensemble Phase III trial, a large-scale multi-country clinical trial last month (September 2020). The trial was set to enroll up to 60,000 volunteers to study the safety and efficacy of a single vaccine dose versus placebo in preventing COVID-19.
AstraZeneca CEO Pascal Soriot. AstraZeneca CEO Pascal Soriot also is leading the company in developing a COVID-19 vaccine as well as antibody treatments for COVID-19. Its vaccine candidate, AZD1222 is an adenovirus-based vaccine that was developed at the University of Oxford and licensed to the company. Last month (September 2020), AstraZeneca paused clinical trials for the vaccine to allow an independent committee to review the safety data of a single event of an unexplained illness that occurred in a Phase III trial in the UK. Since then (as of October 2, 2020), regulators in Japan, the UK, Brazil, South Africa, and India have deemed the trials are safe to resume. The company says it continues to work with the US Food and Drug Administration to facilitate review of the information needed to make a decision regarding resumption of the US trial.
In addition, the company received a $486-million award from the US government for the late-stage development and large-scale manufacturing of the company’s AZD7442, a cocktail of two monoclonal antibodies, for the potential treatment or prevention of SARS-CoV-2, the virus that causes COVID-19. The funding will be used for two Phase III clinical trials and related development activities, including a large-scale manufacturing demonstration project and supply of AZD7442 doses in the US. AstraZeneca also says it plans to supply up to 100,000 doses starting toward the end of 2020 and that the US government can acquire up to an additional one million doses in 2021 under a separate agreement.
4. Moderna CEO Stéphane Bancel. Moderna, a clinical-stage biopharmaceutical company developing mRNA therapeutics and vaccines, has risen from the ranks with its investigational mRNA vaccine candidate, mRNA-1273, which is being studied in a Phase III trial of 30,000 participants in the US. The company has signed multiple agreements for the supply of the vaccine, including with the US government, which is funding both the development and manufacturing of the vaccine. As of July (July 2020), Moderna said it was on track to be able to deliver approximately 500 million doses per year of its COVID-19 vaccine and possibly up to 1 billion doses per year, beginning in 2021, from its internal US manufacturing site and strategic collaboration with Lonza. In addition, Moderna also has a collaboration with Catalent for large-scale, commercial fill–finish manufacturing of mRNA-1273 at Catalent’s biologics facility in Indiana.
5. Gilead Sciences Chairman and CEO Daniel O’Day. Gilead Sciences Chairman and CEO Daniel O’Day is leading the company with the development and supply of a COVID-19 treatment, Veklury (remdesivir), an antiviral drug. Veklury is being evaluated in multiple international Phase III trials for treating COVID-19 in different patient populations and formulations and in combination with other therapies. It has been approved or authorized in approximately 50 countries globally, including in the US through emergency use authorization. The company announced on October 1, 2020, that is now meeting real-time demand for Veklury in the US and anticipates meeting global demand for Veklury this month (October 2020), even in the event of potential future surges of COVID-19. Also, Gilead announced an agreement earlier this month (October 2020) with the European Commission that enables 37 participating countries in the European Union and the European Economic Area as well as the UK to purchase remdesivir for real-time demand and stockpiling needs, coordinated by the European Commission. The agreement covers purchases of remdesivir over the next six months with the option to extend further. In addition, Gilead’s voluntary licensing partners are currently supplying generic remdesivir in more than 40 countries.
6. Regeneron Pharmaceuticals Co-Founder, President and CEO Leonard S. Schleifer. Leonard S. Schleifer, co-founder, President, and CEO of Regeneron Pharmaceuticals, a Tarrytown, New York-based biopharmaceutical company, is another CEO that is leading a company with potential treatments against COVID-19. Last month (September 2020), the company advanced its investigational anti-viral antibody cocktail, REGN-COV2, against COVID-19 to Phase III trials in partnership with the University of Oxford. In July (July 2020), the company received a $450-million award from the US government for the manufacture and supply of the antibody cocktail as part of Operation Warp Speed, a US government initiative to speed the development of COVID-19 vaccines and developments.
7 (tie). CureVac CEO Franz-Werner Haas and Novavax CEO and President Stanley C. Erck. Two CEOs of smaller companies developing COVID-19 vaccines, CureVac CEO Franz-Werner Haas and Novavax CEO and President Stanley C. Erck, make our list of pharma CEOs on the rise. CureVac is a Tübingen, Germany-based clinical-stage biopharmaceutical company developing mRNA therapeutics and is receiving funding from the German government to fund development of its mRNA COVID vaccine candidate. CureVac first began development of its mRNA-based COVID-19 vaccine candidate in January 2020, began a Phase I clinical study in June 2020 at clinical study centers in Germany and Belgium, and initiated a Phase IIa study in Peru and Panama in late September (September 2020). The company has production capacities for mRNA vaccines at its headquarters in Tübingen and is currently expanding those manufacturing capacities to allow for broad-scale manufacturing of other mRNA-based vaccines.
The company conducted an initial public offering earlier this year (2020) and outside of COVID-19 related projects, formed a strategic collaboration agreement with GlaxoSmithKline (GSK), in a deal worth up to £866 million ($1.1 billion), for the research, development, manufacturing and commercialization of up to five mRNA-based vaccines and monoclonal antibodies targeting infectious disease pathogens. Under the deal, GSK also agreed to take a 10% stake in the company.
Novavax CEO and President Stanley C. Erck is also leading the company with the development of its COVID-19 vaccine candidate, NVX CoV2373, a prefusion protein made using Novavax’s recombinant protein nanoparticle technology and includes Novavax’s proprietary saponin-based adjuvant, Matrix M. NVX-CoV2373 is being evaluated in a Phase II trial in the UK and two ongoing Phase II studies that began in August (August 2020). Novavax, based in Gaithersburg, Maryland, has secured $2 billion in funding for its global coronavirus vaccine program, including up to $388 million in funding from the Coalition for Epidemic Preparedness Innovations (CEPI), a public-private partnership.
9. Seagen (formerly Seattle Genetics) President, CEO and Chairman Clay Siegall. Outside of COVID-19-related projects, Seattle Genetics, recently renamed as Seagen, is a Bothell, Washington-based biopharmaceutical company well established for its position in antibody drug conjugates (ADCs). Last month (September 2020), the company formed a collaboration with Merck & Co., in a deal worth up to $4.2-billion, for Seattle Genetics’ ladiratuzumab vedotin, for treating breast cancer and other solid tumors. The deal includes a $600-million upfront payment by Merck, a $1.0-billion equity investment by Merck into Seagen, and $2.6 billion in potential milestones.
Seagen’s lead commercial product is Adcetris (brentuximab vedotin), an ADC for treating relapsed classical Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma and is approved in over 70 countries and which posted 2019 sales of $628 million. Over the last year, Seagen has launched two new cancer medicines: Padcev (enfortumab vedotin-ejfv) for treating bladder cancer and Tukysa (tucatinib) for treating advanced breast cancer.
10. Genmab CEO Jan G. J. van de Winkel. In June (June 2020), Genmab, a Copenhagen-based biopharmaceutical company specializing in antibody therapeutics for treating cancer, signed a broad collaboration agreement with AbbVie, worth up to $3.9 billion ($750 million up front with the potential to earn $3.15 billion in milestone payments), to jointly develop and commercialize three of Genmab’s early-stage investigational bispecific antibody product candidates and for a discovery research collaboration for future differentiated antibody therapeutics for cancer.
The deal with AbbVie is the latest for the company, which has deals with other large pharma companies, including J&J, Roche, and Novartis.






