Interchangeability Between Hard Gelatin and Hard Hypromellose Capsules Without Gelling Agent
Purpose
Hard gelatin capsules have long been used in a substantial proportion of oral dosage forms. However, they have some shortcomings. Concerns include the potential to become cross-linked, resulting in problems with dissolution, and the growing consumer preference for non-animal products due to dietary preferences as well as concerns about bovine spongiform encephalopathy. Hypromellose (hydroxypropyl methylcellulose [HPMC]), which is derived from cellulose and is less likely to cross-link, is the most common option for replacing gelatin in hard capsules. Capsules made with HPMC also have many other beneficial properties, including excellent mechanical strength and machinability.
The objective of this study was to demonstrate interchangeability between hard gelatin capsules (gelatin capsules) and hard HPMC capsules without gelling agent (HPMC capsules). In the USP dissolution apparatus, drug dissolution occurs in an environment that is different than that of the human gastrointestinal tract. In contrast, the FloVitroTM dissolution test is a biorelevant in vitro test that reflects the physiological environment in the test conditions. The FloVitroTM method uses a flow-through approach that utilizes a series of solid transfer cells that incorporate pharmacokinetic characteristics. Experiments were performed to collect data to: (1) define the dissolution behavior of propranolol HCl (a BCS Class I API) encapsulated into gelatin capsules and HPMC capsules using both a modified USP apparatus II and the FloVitroTM methods, and (2) compare the pharmacokinetic parameters for the gelatin and the HPMC capsules determined from the drug concentration versus time profiles generated by the FloVitroTM method.
Methods
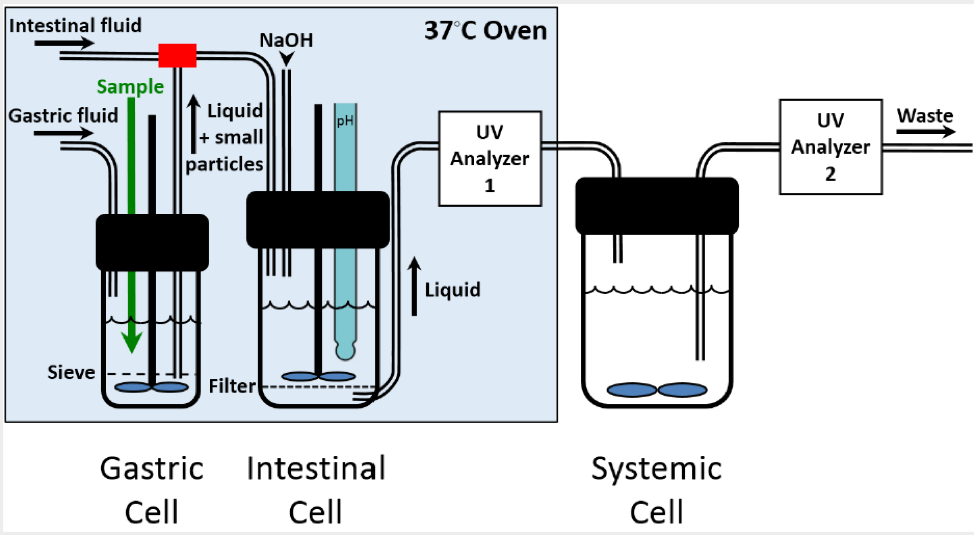
Commercially available empty hard gelatin capsules and empty hard HPMC capsules from CapsCanada® were used in this study. All capsules were clear and transparent without any color additives, and they were filled with 80 mg of propranolol HCl with no excipients. The USP dissolution test was performed using the Distek 2100 apparatus with paddles and stationary hanging baskets, a pH 1.2 dissolution media and a spectrophotometer (Agilent 8453) for the determination of propranolol HCl. The FloVitroTM method was performed using three connected cells containing dissolution media representing gastric, intestinal, and systemic environments in an open-loop configuration (see Figure 1). Samples were collected from the third cell at intervals of 0.5 minutes and propranolol HCl assay was performed by UV /Vis absorption at 290 nm. Similarity between dissolution profiles was evaluated by determining the similarity factor, f2. Two dissolution profiles are considered similar if f2 is greater than 50. Pharmacokinetic parameters generated by the FloVitroTM method were used for the assessment of bioequivalence between propranolol HCl in gelatin and HPMC capsules.
Figure 1. FloVitro™ Technology: three cell system for dissolution testing
Results
USP apparatus II dissolution test results
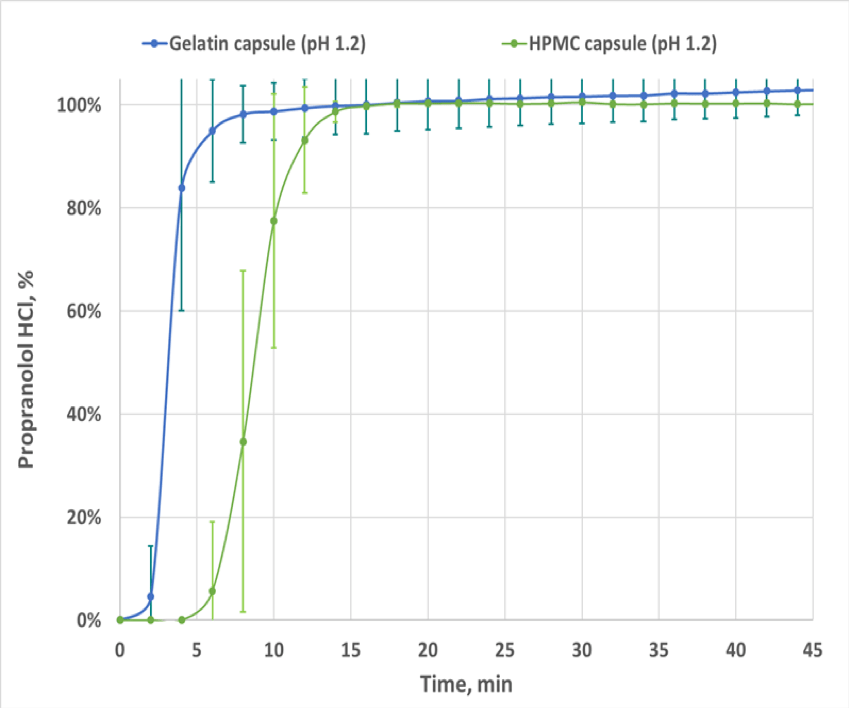
Figure 2. Dissolution Profiles for 80 mg Propranolol HCl Filled in Gelatin (N=6) and HPMC (N=6) Capsules
FloVitroTM dissolution test results
Figure 3. Percentage Recovered Versus Time Data Generated by FloVitroTM Method for Propranolol HCl 80 mg Gelatin (N=6) and HPMC (N=5) Capsules
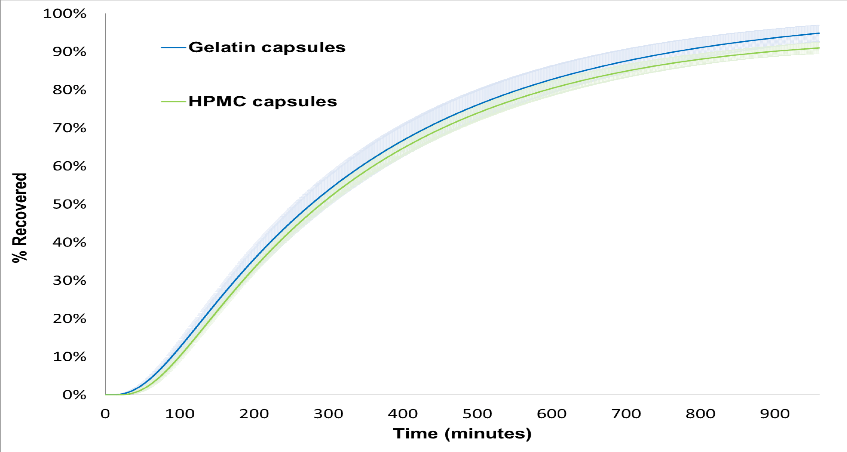
Table 1. Means and Standard Deviations of Pharmacokinetic Parameters for Propranolol HCl 80 mg Gelatin (N=6) and HPMC (N=5) Capsules Subjected to FloVitroTM Dissolution Testing
| Sample | Cmax (mg/L) | tmax (min) | AUC0-16 mg.hr/l | AUC0-inf mg.hr/l | T1/2 (hr) | Kel (hr-1) |
| Gelatin capsules | 33.6a (0.9)b | 127.3 (6.2) | 221.1 (16.0) | 235.7 (21.6) | 3.8 (0.4) | 0.19 (0.02) |
| HPMC capsules without gelling agents | 33.3 (1.4) | 135.3 (7.0) | 210.9 (15.0) | 220.3 (21.0) | 3.0 (1.1) | 0.3 (0.18) |
Ninety percent confidence intervals on the ratio of geometric means for Cmax, AUC0-16, and AUC0-inf of the gelatin and the HPMC capsules were calculated and compared to the bioequivalence interval of 80-125%; the confidence intervals for all three parameters fell within the bioequivalence interval.
Conclusions
When the dissolution testing was performed using the FloVitroTM method under biorelevant conditions, the initial delay in dissolution of propranolol HCl seen in the traditional dissolution testing (USP apparatus II) that resulted in dissimilarity (f2 < 50) was not seen and as determined by the f2 similarity factor the dissolution profiles of propranolol HCl gelatin and HPMC capsules were found to be similar (see Figures 2 and 3). Demonstration of bioequivalence by determining the 90% confidence intervals on the ratio of the key pharmacokinetic parameters, Cmax, AUC0-16 and AUC0-inf, determined using the concentration versus time curves generated by the FloVitroTM method indicates propranolol HCl filled in gelatin and HPMC capsules have comparable pharmacokinetic properties (see Table 1). The results from this study indicate the hard gelatin and the hard HPMC capsules made without gelling agents are interchangeable in immediate-release applications.

CapsCanada®, a Lyfe Group Co., is one of the world’s leading manufacturers of high-quality empty hard capsules. The company offers customized solutions that solve complex product development challenges for the global pharmaceutical, nutraceutical, and consumer health industries. With a presence in over 60 countries, CapsCanada also offers the advantages of strategic global distribution locations and full-service representation.