SK pharmteco Launches Newly Formed CDMO
 |
|
Aslam Malik |
SK Holdings, the holding company of South Korea’s SK Group, has launched a new organizational structure and identity for its contract development and manufacturing organization (CDMO) business, SK pharmteco. Aslam Malik, the recently appointed CEO of SK pharmteco, outlined the new organizational structure and operations of the CDMO at the DCAT Member Company Announcement Forum—Virtual Edition, a special online forum of the major news announcements from DCAT member companies originally to be made March 23, 2020 at DCAT Week ’20 in New York.
The formation of SK pharmteco, which took effect in January (January 2020), involved a governance restructuring, which combined SK biotek in Korea and SK biotek in Ireland, along with AMPAC Fine Chemicals in the US, to form SK pharmteco, the consolidated entity (see Figure 1). Malik, who was the CEO of AMPAC Fine Chemicals, is leading SK pharmteco as CEO.
SK pharmteco is headquartered in Sacramento, California, and it is a product of two key recent acquisitions by SK to build its CDMO business. In 2018, SK acquired AMPAC Fine Chemicals a Rancho Cordova, California-headquartered contract manufacturer of active pharmaceutical ingredients (APIs) and intermediates with operations in Rancho Cordova and El Dorado Hills, California; La Porte, Texas; and Petersburg, Virginia.
Also in 2018, SK biotek, the life-sciences arm of SK for custom chemical development, advanced intermediates and API manufacturing, completed its acquisition of an API manufacturing launch site in Swords, Dublin, Ireland from a leading pharmaceutical company. The transaction included the transfer of approximately 350 employees to SK and the addition of a 21-acre site with 82 cubic meters of reactor capacity.
In addition to the Swords facility and the facilities of AMPAC Fine Chemicals, SK’s CDMO business includes two production facilities, respectively in Sejong and Daejeon, South Korea.
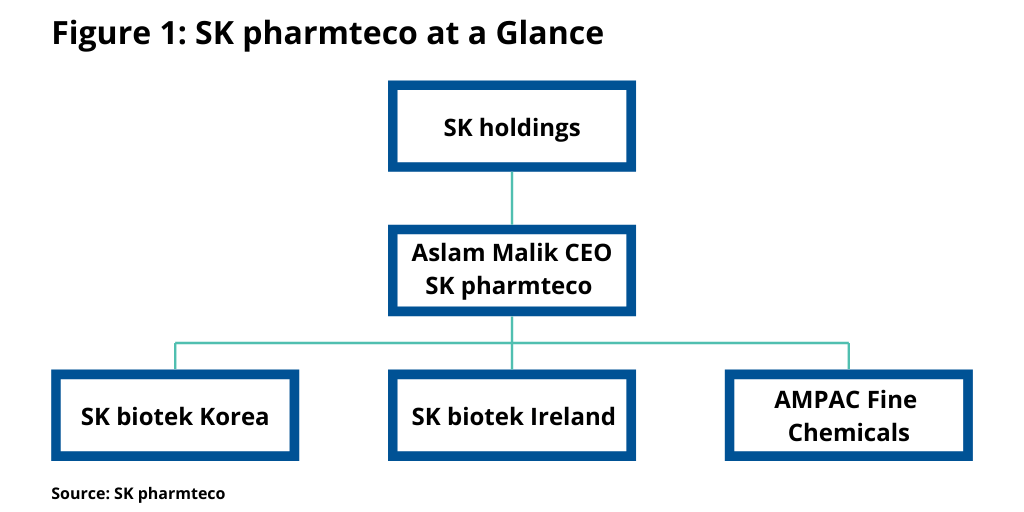
SK pharmteco (see Figure 1) is now the consolidated entity of three business units: AMPAC Fine Chemicals, SK biotek Ireland in Dublin, and SK biotek in Korea. The new company has four facilities in North America (from AMPAC Fine Chemicals): a dedicated analytical services operation in California and three API production facilities in: Rancho Cordova and El Dorado Hills, California; LaPorte, Texas; and Petersburg, Virginia. There is also an API facility in Swords, Ireland (from SK biotek in Ireland) and two additional facilities, respectively in Sejong and Daejeon, Korea (from SK biotek in Korea). The combined capacity is currently 1,020 cubic meters with room for expansion. The company says that additional investment is planned to add further capabilities in particle engineering, continuous processing, and batch chromatography.
Malik says that with the formation of SK pharmteco and the new organizational structure, a number of new positions and appointments have occurred. For example, each facility now has a dedicated site manager reporting into the head of its business unit. The company is also standardizing its global project teams to ensure clients have a dedicated project manager working alongside them. Other standardization includes the formation of global organizations in legal, procurement, finance, and research and development.

