FDA Issues New Guidance on Remote Inspections
Last week (April 14, 2021), the FDA issued final guidance on remote inspections, which the FDA has used in lieu of onsite inspections due to COVID-19 restrictions. The FDA is outlining what to expect in remote inspections, including how to prepare, how such inspections are conducted, and how the agency deals with findings from the inspections.
The FDA and remote inspections
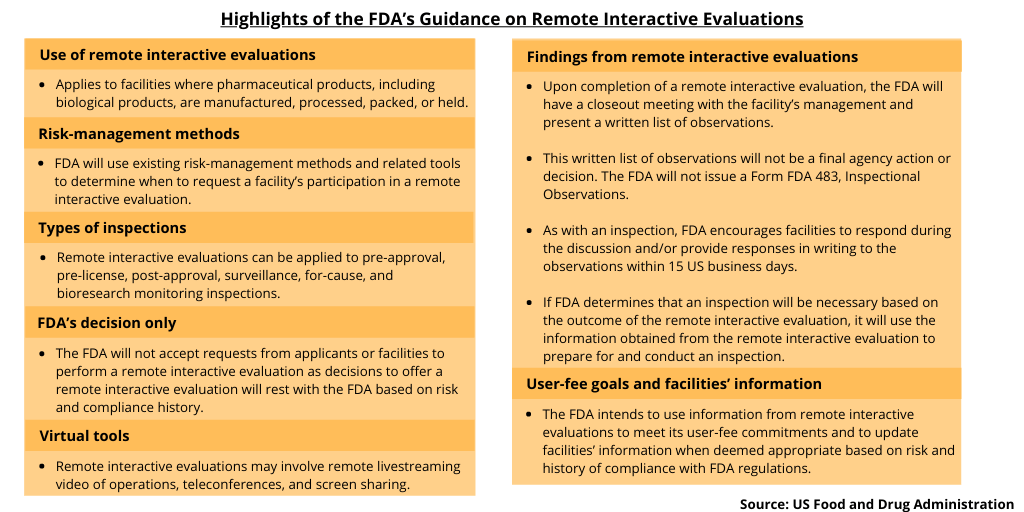
During the COVID-19 pandemic, the US Food and Drug Administration (FDA) has used a variety of tools to oversee facilities that manufacture FDA-regulated products. These tools include record requests in advance of or in lieu of a drug-facility inspection, relying on information from trusted regulatory partners, and remote interactive evaluations (such as remote livestreaming video of operations, teleconferences, and screen sharing). The FDA has used some or all of these approaches to evaluate facilities when inspections of drug facilities were not possible due to travel or quarantine restrictions. Last week (April 14, 2021), the agency issued new guidance, Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities During the COVID-19 Public Health Emergency, to provide further clarity for regulated facilities on how the FDA will request and conduct these remote interactive evaluations and use the findings from these evaluations.
“We recognize that remote interactive evaluations do not replace inspections, and that there are situations where only an inspection is appropriate based on risk and history of compliance with FDA regulations,” said the FDA in an April 14, 2021 statement. “Within the exceptional context of a global pandemic, we see remote interactive evaluations as part of a necessary strategy to evaluate medical product facilities by using all available approaches to ensure the medical products we regulate are safe, effective and of high quality.”
Snapshot: inside the new guidance
The guidance describes various interactive and virtual tools used in a remote interactive evaluation and how remote interactive evaluations will be requested by the FDA and conducted for the duration of the COVID-19 public health emergency at any facility where pharmaceutical products, including biological products, are manufactured, processed, packed, or held. The guidance also applies to facilities covered under the FDA’s bioresearch monitoring program and to outsourcing facilities registered under Section 503B of the Federal Food, Drug, and Cosmetic Act.
The FDA says that it will use existing risk-management methods and related tools to determine when to request a facility’s participation in a remote interactive evaluation. The FDA conducts inspections for many purposes and programs, including pre-approval and pre-license, post-approval, surveillance, for-cause, and bioresearch monitoring programs. The agency will consider each of these inspectional program areas as possible candidates for remote interactive evaluations but also recognizes that there will be instances where only an onsite inspection will be appropriate.
The FDA intends to use information from remote interactive evaluations to meet its user-fee commitments and to update facilities’ information when deemed appropriate based on risk and history of compliance with FDA regulations. Facilities can choose to decline the FDA’s request to perform a remote facility evaluation; however, this may delay the agency’s ability to evaluate the facility or product and make a regulatory decision. The FDA will not accept requests from applicants or facilities to perform a remote interactive evaluation as decisions to offer a remote interactive evaluation will rest with the FDA based on risk and compliance history.
Industry feedback
The Pharmaceutical Research and Manufacturers of America (PhRMA), which represents innovator and research-based bio/pharmaceutical companies, is supportive of the new guidance.
“The US Food and Drug Administration (FDA) guidance on remote interactive evaluations is an important step from the Agency in describing actions it may take to evaluate and oversee manufacturing facilities and enable timely regulatory decision-making,” said Dr. Richard Moscicki. PhRMA’s Executive Vice President of Science and Regulatory Advocacy and Chief Medical Officer, in an April 15, 2021 statement. “The biopharmaceutical industry recognizes that the COVID-19 pandemic has presented novel challenges to FDA in its regulatory oversight of manufacturing facilities. We applaud the FDA for adapting to these unique challenges and taking steps to describe the ‘remote interactive evaluations’ the Agency may conduct to facilitate regulatory decision-making and ensure timely access to new, innovative treatments for patients.”
PhRMA pointed to four elements of the new guidance that it deemed as particularly important as outlined below.
User-fee goals. The guidance provides explicit recognition that the use of remote interactive evaluations will generally enable FDA to meet its user-fee goal dates.
Virtual tools. The guidance outlines the use of virtual technologies to facilitate FDA’s oversight of manufacturing facilities and regulatory decision-making.
Communication with manufacturing facilities. The guidance provides recognition that discussion with manufacturing facilities on logistics, responsibilities, and expectations is critical to enabling remote interactive evaluations.
Actions following a remote interactive evaluation. The guidance also outlines the actions that FDA will take to conclude a remote interactive evaluation and how the agency may use information obtained during such an evaluation.
Preparing for a remote interactive evaluation
Once a facility confirms its willingness and ability to participate in a remote interactive evaluation, the FDA will schedule a brief virtual meeting to discuss logistics, responsibilities, and expectations. The guidance outlines the discussion topics as part of that initial virtual meeting as outlined below:
- Goals. Objectives and scope of the remote interactive evaluation;
- FDA team. Introduction of the FDA remote interactive evaluation team and the remote interactive evaluation lead;
- Facility contacts. Identification of the facility point of contact and all other participants (e.g., sponsor or contract research organization, monitor, remote ancillary operations);
- Schedule and duration. Schedule of virtual interactions and the anticipated duration of the remote interactive Evaluation;
- FDA’s expectations. FDA’s expectations during the livestreaming walkthroughs of the facility;
- Times for remoted interactive evaluations. Time zone differences and translation services (i.e., spoken and written translation), if applicable. Virtual interactions, including remote observation of manufacturing operations or livestream assessment of data, usually will occur during the facility’s normal business hours;
- Information-sharing. Methods for sharing requested information, including sharing documents and the use of video-streaming technology;
- Technical issues. Technological limitations that could impair or prevent FDA’s remote interactive evaluation of the facility; and
- Internet connectivity. Check of the Internet connection throughout the facility to verify that the signal strength is adequate to support livestreaming video and audio during the actual remote interactive evaluation.
Conducting a remote interactive evaluation
The guidance also outlines what the FDA may do as part of a remote interactive evaluation as outlined below:
- Document requests. Request and review documents, records, and other information (electronic systems);
- Virtual tools. Use livestream and/or pre-recorded video to examine facilities, operations, and data and other information;
- Interviews. Through the facility’s point of contact, schedule interviews and meetings to address any questions or concerns;
- Corrective actions. Evaluate a facility’s corrective actions (e.g., in response to a previous inspection or evaluation, or to the current remote interactive evaluation). An inspection instead of a remote interactive evaluation may be necessary to verify the adequacy of some corrective actions, or if evaluating the corrective actions remotely would unreasonably extend the duration of the remote interactive evaluation; and
- Observations. Provide verbal updates to the facility on observations and outstanding issues, whenever feasible.
Findings from a remote interactive evaluation
Upon completion of a remote interactive evaluation, the FDA will have a closeout meeting with the facility’s management. During this meeting, the FDA will usually present a written list of observations, if any, and describe and discuss any observations in sufficient detail to enable understanding and foster an appropriate response. This written list of observations will not be a final agency action or decision. The FDA will not issue a Form FDA 483, Inspectional Observations. As with an inspection, the FDA encourages facilities to respond during the discussion and/or provide responses in writing to the observations within 15 US business days.
The guidance specifies that depending on the purpose and outcome of the remote interactive evaluation, the information and documentation collected may be used to, among other regulatory purposes:
- Support the FDA’s assessment of pending applications, including whether to approve an Application;
- Preclude the need for an inspection in follow-up to a reported concern or defect;
- Support a regulatory meeting, Warning Letter, import alert, recall activities, or enforcement action;
- Rank or prioritize a facility for an inspection, particularly a surveillance cGMP Inspection; and
- Justify a follow-up or compliance inspection or any other surveillance activity.
After the remote interactive evaluation concludes, the FDA will provide a copy of the final remote interactive evaluation report to the facility. A remote interactive evaluation report and any written list of observations may be subject to a disclosure request under the Freedom of Information Act.
If the FDA determines that an inspection will be necessary based on the outcome of the remote interactive evaluation, the agency will use the information obtained from the remote interactive evaluation to prepare for and conduct the inspection.






