FDA Updates Risk Mitigation for Nitrosamine Impurities
The FDA has provided updates on mitigation strategies to reduce the risk of nitrosamine drug substance-related impurities in drug products. What does the agency suggest?
The issue of nitrosamine impurities, possible carcinogens, in certain pharmaceutical products first emerged in the industry three years ago, and was unusual in that it surfaced in certain generic drugs that had established manufacturing processes without prior incidents. Since then, along with voluntary company recalls of certain products, regulators in the US and European Union have issued guidelines for testing of products to detect nitrosamine impurities, acceptable daily intake levels, and risk evaluation and assessment. Last month (November 2021), the FDA provided drug manufacturers with additional points to consider in developing nitrosamine-mitigation strategies, including consideration of the role of formulation design for controlling nitrosamine levels in drug products.
Nitrosamine impurities: a continuing issue
The issue of nitrosamine impurities first surfaced in 2018 when the US Food and Drug Administration (FDA) and the European Medicines Agency initiated investigations of nitrosamine impurities in certain “sartan”-containing APIs, used in anti-hypertensive and cardiovascular drugs such as valsartan candesartan, irbesartan, losartan, and olmesartan. They later broadened those investigations to include prescription and over-the-counter forms of ranitidine, a H2 (histamine-2) blocker used to decrease the amount of acid created by the stomach, and metformin extended-release products, used to treat Type II diabetes. As part of their investigations, the regulatory agencies issued guidelines for testing of products to detect nitrosamine impurities, acceptable daily intake levels, and risk evaluation and assessment. Companies also issued voluntary recalls of products found or potentially having levels of these impurities above acceptable intake levels.
The FDA issues guidance
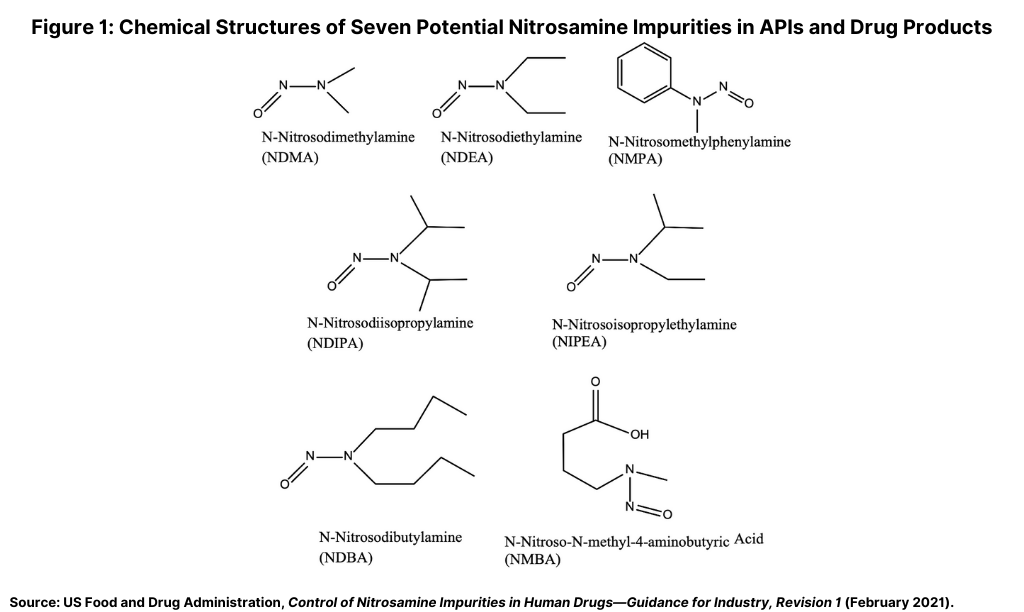
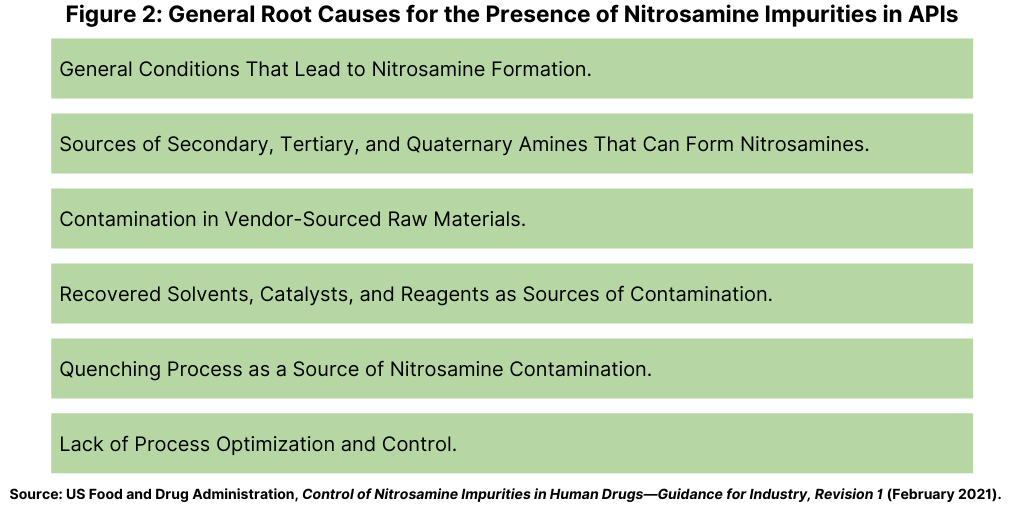
As part of developing risk-mitigation approaches for the industry, the FDA issued a guidance for industry, Control of Nitrosamine Impurities in Human Drugs, in September 2020 to recommend steps manufacturers of APIs and drug products should take to detect and prevent objectionable levels of nitrosamine impurities in pharmaceutical products. Figure 1 (see above) includes some examples of these impurities. The guidance and a subsequent updated guidance issued in February 2021 further described conditions that may introduce nitrosamine impurities (see Figure 2) and described a three-step mitigation strategy.
The updated guidance from February 2021 specified timeframes for completion of nitrosamine-mitigation activities by drug manufacturers. The strategy includes risk assessment (Step 1), confirmatory testing if risks are identified (Step 2), and reporting changes implemented to prevent or reduce the presence of nitrosamine impurities in drug products in approved and pending new drug applications and abbreviated new drug applications (Step 3).
Timelines for implementing nitrosamine-mitigation measures
Under the FDA guidance, risk assessments (Step 1) were to have been completed by the recommended completion date of March 31, 2021. FDA’s recommended timeline for completion of confirmatory testing and reporting changes (Steps 2 and 3) is October 1, 2023. With the risk-assessment stage completed and approximately two years left for manufacturers to implement changes, the FDA is providing manufacturers with additional points to consider in developing nitrosamine-mitigation strategies, including consideration of the role of formulation design for controlling nitrosamine levels in drug products.
Excipients as a possible cause of nitrosamine impurities
Recently, the FDA said it has received additional reports of certain types of nitrosamine impurities that formed in several drug products. These nitrosamine drug substance-related impurities (NDSRIs) are a class of nitrosamines sharing structural similarity to the API. NDSRIs can be generated during manufacturing or during the shelf-life storage period of the drug product. In some cases, the root cause of NDSRI formation has been attributed to nitrite impurities present in excipients at parts-per-million amounts. Nitrite impurities have been observed in a range of commonly used excipients (as well as water) and may lead to the formation of NDSRIs in certain drug products, noted the FDA in a November 2021 agency communication on the subject.
The FDA expects manufacturers to ascertain the presence of nitrosamines, including NDSRIs, using the three-step mitigation strategy described in the agency’s guidance. The FDA communication notes that as manufacturers should now be engaged in the confirmatory testing and reporting changes stages of their mitigation activities (Steps 2 and 3), if NDSRIs are detected in the drug product at objectionable levels, the FDA is encouraging applicants to develop control strategies and/or design approaches to reduce NDSRIs to acceptable levels.
Mitigation strategies in drug formulations
One mitigation strategy described in the FDA’s guidance includes a supplier-qualification program that takes into account potential nitrite impurities across excipient suppliers and excipient lots to reduce the risk of nitrosamine formation in the drug product. However, the FDA noted that this is only one strategy, and other approaches may be equally or more effective in controlling nitrosamine levels. Therefore, the FDA is encouraging manufacturers to explore other approaches to mitigate or prevent formation of NDSRIs. Examples of possible mitigation strategies related to formulation design are described below.
The first of these possible mitigation strategies is derived from literature reports showing that commonly used antioxidants, such as ascorbic acid (vitamin C) or alpha-tocopherol (vitamin E), inhibit the formation of nitrosamines in vivo based upon data from human gastric fluid in vitro studies. The FDA noted in its communication that recent work has employed this concept in formulation design and has preliminarily demonstrated that the addition of antioxidants to formulations may significantly inhibit the formation of NDSRIs in drug products.
A second possible approach is based upon the fact that the formation of nitrosamines typically occurs under acidic conditions, whereas in a neutral or basic environment, the kinetics of these reactions are significantly reduced. Thus, formulation designs that incorporate excipients, such as sodium carbonate that modify the micro-environment to neutral or basic pH, should in principle inhibit the formation of NDSRIs, noted the FDA in its communication. The FDA says that each manufacturer should determine the potential benefit from and demonstrate the suitability of any reformulation approach.
The FDA says that manufacturers should consider these as well as other innovative strategies to reduce the formation of NDSRIs to acceptable levels in drug products. The FDA says it will consider meeting requests, as appropriate, to discuss innovative mitigation strategies with prospective applicants or manufacturers. Applicants may also choose to submit formulation changes through supplements or amendments to their applications without scheduling a meeting with the FDA, and any manufacturer, including non-application drug manufacturers, may implement any formulation changes in accordance with appropriate cGMP regulations and related guidance.






