CordenPharma Adds Commercial Aseptic Fill–Finish Capacity and R&D Lab
 |
|
Dr. Stephen Houldsworth |
CordenPharma is proceeding with an approximate EUR 30-million ($33-million) investment at its CordenPharma Caponago injectable drug-product facility in Italy. Dr. Stephen Houldsworth, Vice President, Global Head of Platform Management & Marketing, CordenPharma International, provided an update in the DCAT Member Company Announcement Forum—Virtual Edition, a special online forum of the major news announcements from DCAT member companies originally to be made March 23, 2020 at DCAT Week ’20 in New York.
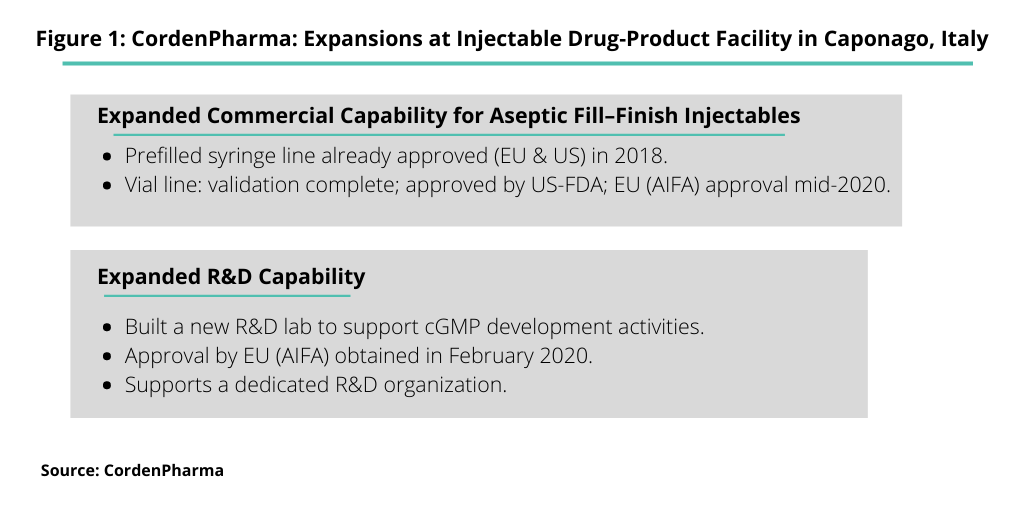
Houldsworth detailed the company’s three-phase capital-expenditure program to add commercial-scale capacity for aseptic fill–finish injectables and expanded R&D capabilities at the company’s injectables drug-product facility in Caponago, Italy (see Figure 1), with certain key milestones upcoming in 2020 and beyond. The expansions are outlined below.
- Phase 1 of the expansion program (completed in 2017) saw the introduction of aseptic fill–finish capability with the construction and qualification of a clinical/development plant.
- Phase 2a of the program (completed in 2018) saw the construction of an aseptic prefilled syringe and cartridge line, which has undergone regulatory approvals in the US and European Union.
- Phase 2b further saw the expansion of aseptic processing capabilities with the construction of a second filling line dedicated solely to vial production. Construction was completed, and the line received approval by the US Food and Drug Administration in 2019, with further approval by the Italian Medicines Agency (AIFA) expected by mid-2020. Completion of a lyophilized drug-product vial line is expected by the end of the year (2020), with approvals expected in early 2021.
- Phase 3 of the expansion saw the completion of the plan with the construction and regulatory approval of a dedicated R&D laboratory.
The company’s new R&D laboratory supports injectable drug-product development of a broad spectrum of molecule types (small molecules, monoclonal antibodies, antigen-binding fragments, peptides, oligonucleotides) from clinical formulation development to late-stage development, including technology transfer of existing products. The lab also supports a number of drug-product forms (solutions, emulsions and freeze-dried solids) in combination with different presentations (vials, prefilled syringes and cartridges).
Houldsworth explained that the expansion is consistent with a broader corporate strategy of offering fully integrated supply (active pharmaceutical ingredients, drug products, packaging, and logistics) for the development and manufacturing of not only injectables, but also drugs for various therapeutic applications across all of CordenPharma’s technology platforms.


