Recipharm Begins Integration of $629-M Acquisition of Consort Medical
 |
|
Mark Quick |
Recipharm is building its capabilities and scale following the completion of its £505 million ($629 million) acquisition of Consort Medical, a CDMO of active pharmaceutical ingredients (APIs), drug products, and drug devices. Mark Quick, Executive Vice President of Corporate Development of Recipharm and the newly appointed CEO of Consort Medical, provided an update of the newly combined company at the DCAT Member Company Announcement Forum—Virtual Edition, a special online forum of the major news announcements from DCAT member companies originally to be made March 23, 2020 at DCAT Week ’20 in New York.
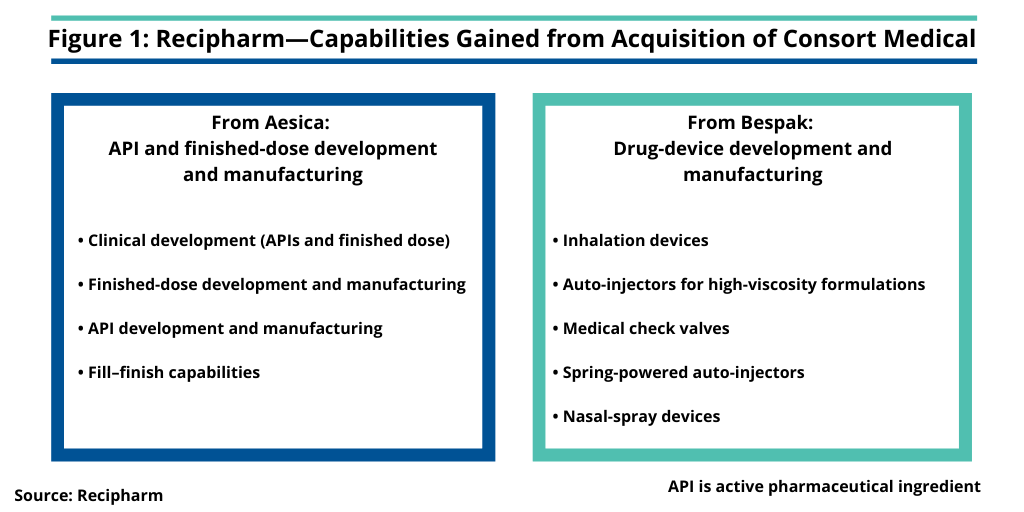
Quick explained that he took over his new role as CEO of Consort in addition to his role as Executive Vice President of Corporate Development of Recipharm to lead the Consort Medical business through the transition and integration of it into Recipharm following the completion of the acquisition in February 2020. Consort Medical is the parent company of Aesica, a CDMO of APIs and drug products, and Bespak, a CDMO of drug devices (see Figure 1).
With the acquisition, Recipharm gains Consort’s 2,000 employees and has added 10 facilities across the UK, Italy, and Germany. Of the approximately 2,000 people globally from Consort Medical, approximately 1,400 are located in the UK. Consort has UK facilities in King’s Lynn, Cambridge, Nelson, Milton Keynes, Cramlington, Queensborough and Hemel Hempstead. It has facilities in Germany in Monheim and Zwickau and a facility in Pianezza, Italy. The acquisition adds to Recipharm’s existing development and manufacturing network, which includes facilities in France, Germany, India, Israel, Italy, Portugal, Spain, Sweden, the UK and the US. Overall, the newly combined company has more than 30 facilities globally in Europe, the US, Israel, and India.
Recipharm’s Quick says the acquisition positions Recipharm as one of the top five global CDMOs with sales of more than $1billion (SEK 10 billion) with an integrated offering of API development and manufacturing, formulation development and finished-dose manufacturing, and device development and manufacturing. The combined company has more than 9,000 employees and a project portfolio of more than 500 products with more than 2,700 units of presentations of various dosage forms.
Quick says that Recipharm will support Consort’s Bespak Division as a stand-alone business while the Aesica Division will be integrated into Recipharm’s current CDMO business, which will add to Recipharm’s development and manufacturing capabilities for both drug substances and drug products.
Aesica adds to Recipharm’s existing capabilities in API development and manufacturing and drug-product formulation development and manufacturing (see Figure 1). The addition of Bespak’s drug-device development and manufacturing capabilities enhances Recipharm’s existing inhalation-drug capabilities. In 2018, Recipharm acquired Sanofi’s inhalation contract manufacturing business, including a manufacturing facility located in Holmes Chapel, UK. That facility provides development and manufacturing capabilities for novel respiratory products and complemented the inhalation development expertise offered by the Recipharm team in Research Triangle Park, North Carolina. The addition of Bespak adds capabilities in development and manufacturing of inhalation devices. Bespak also has development and manufacturing capabilities for auto-injectors and nasal-spray devices (see Figure 1).


